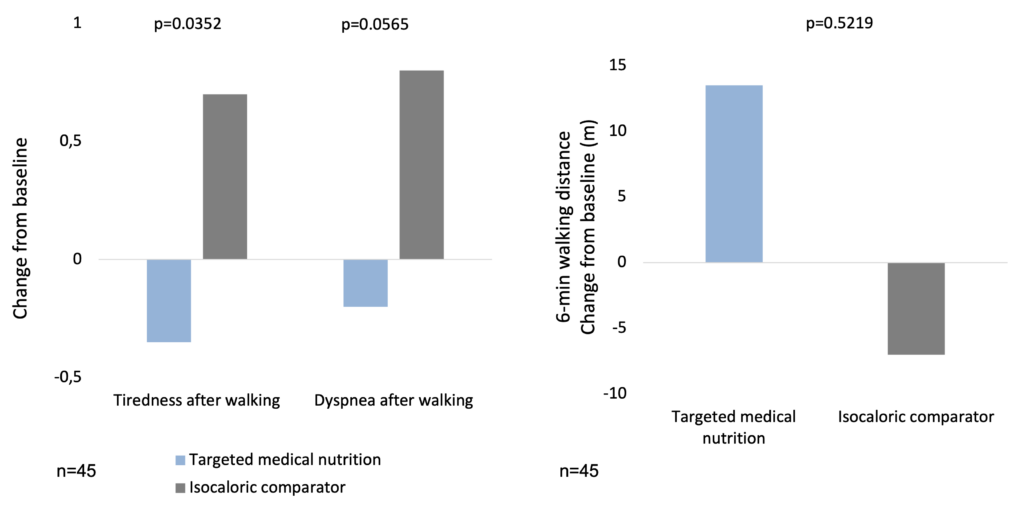MediDrink Pulmo
Disease-related malnutrition
Disease-related malnutrition (DRM) has been an important and under-recognized problem for many years, affecting about 20 million patients in the EU and 33 million patients in all European countries (Ljungqvist et al. 2009, Ljungqvist et al. 2010). DRM is usually caused by the joint action of an underlying disease and dietary deficiency (Naber et al. 1997). As a consequence, treatment should focus not only on the disease itself, but also on nutritional intervention.
Malnutrition refers to deficiencies, excesses, or imbalances in a person’s intake of energy and/or nutrients. One type of malnutrition is undernutrition, which includes stunting (low height for age), wasting (low weight for height), underweight (low weight for age) and micronutrient deficiencies or insufficiencies (a lack of important vitamins and minerals). Malnutrition affects people in every country. Around 1.9 billion adults worldwide are overweight, while 462 million are underweight. An estimated 41 million children under the age of 5 years are overweight or obese, while some 159 million are stunted and 50 million are wasted (WHO 2016).
Malnutrition is as much a cause as a consequence of ill health: a poor food intake, especially for a prolonged period, makes patients more prone to illness and injury that can lead to a reduced appetite through a wide variety of mechanisms – which again results in poor food intake. Thus, it is a vicious cycle which often requires medical help to stop.
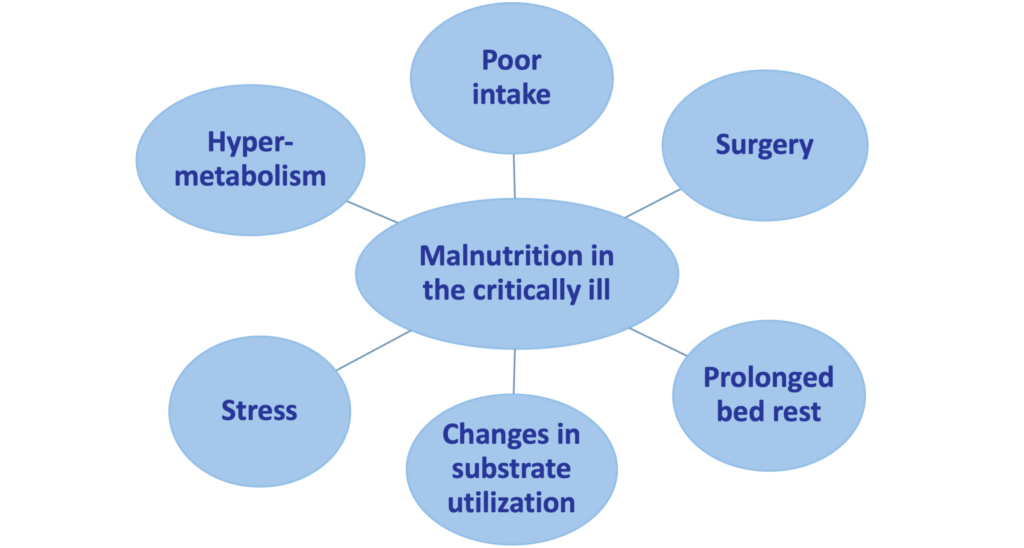
Factors contributing to the development of malnutrition in the critically ill.
Disease-related malnutrition (malnutrition caused by the changes of the body metabolism which increases the daily nutritional needs due to illness) has been an important and under-recognized problem for many years, and it continues to be a growing major public health problem with an aging population (Correia et al. 2003, Meijers et al. 2009). Since disease-related malnutrition inversely affects different organs and systems of the body and leads to severe physical and psycho-social consequences, all health care settings need to develop ways for the early diagnosis and the treatment / prevention of this condition. Surveys from the United Kingdom (UK) suggest that the majority of persons affected by or at risk of malnutrition are not those being treated in the hospital. Of the estimated 3 million people affected by malnutrition in the UK, only 2% are in hospitals, 93% live in the community, largely in their homes, 2-3% in sheltered housing, and 5% in care homes (Pryke et al. 2013). In the European Union (EU) countries, about 20 million patients are affected by disease-related malnutrition. This number is as high as 33 million patients when all countries of Europe are considered. Annual treatment costs of disease-related malnutrition reach € 120 billion in the EU, and about € 170 billion in Europe (Ljungqvist et al. 2009, Ljungqvist et al. 2010). In Germany, UK, and Ireland, the annual costs of disease-related malnutrition on a national level have been calculated: € 9 billion in 2006, and € 15 billion in 2007 (Freijer et al. 2014a, Freijer et al. 2014b).
Nutritional depletion in Western countries is usually caused by the joint action of an underlying disease (e.g. cancer, chronic obstructive pulmonary disease [COPD], inflammatory bowel disease [IBD], cognitive impairment of the elderly, Alzheimer disease) and dietary deficiency (Naber et al. 1997). As a consequence, treatment should focus not only on the disease itself, but also on nutritional intervention. The most common diseases that can cause malnutrition include oncological diseases such as cancer, pulmonary diseases such as chronic obstructive pulmonary disease or cystic fibrosis, and gastroenterological diseases such as inflammatory bowel disease. Certain treatments of these diseases, such as chemotherapy or radiation therapy, can also have a negative impact on nutrition.
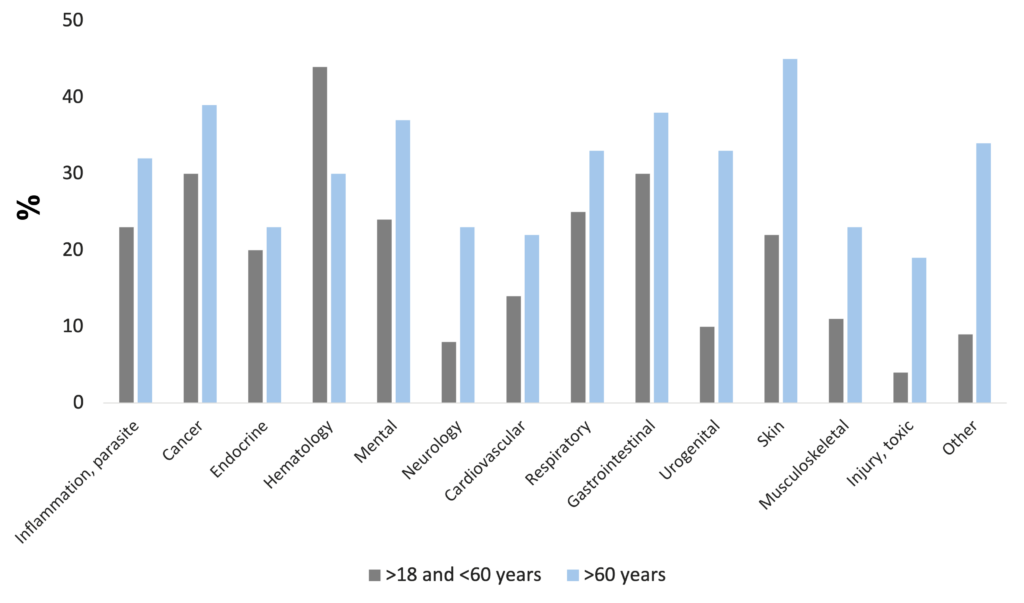
Disease-related malnutrition is present in a wide range of diseases, e.g. in infectious and parasitic diseases, oncologic, endocrine, gastrointestinal, pulmonary, hematologic, psychiatric, urogenital, and neurological disorders. The problem is known worldwide, affecting about 20 million patients with the cost of 120 billion Euros in the EU countries (Freijer et al. 2013).
Frequency of malnutrition in different diseases (Freijer et al. 2013)
For patients with a variety of diagnoses, reports indicate that up to 62% may be considered at risk of malnutrition or frankly malnourished on admission to hospital with rates of up to 12.5% in the community. Studies specifically in general medical patients indicate a possible prevalence rate of up to 56% on admission to hospital (Stratton et al. 2003).
The consequences of malnutrition, if left untreated, are serious, causing a marked decline in physical and psychological health and function. Malnutrition has a negative effect on recovery from disease, treatment efficacy, wound healing, frequency of complications (e.g. infection, decubitus), prognosis, mortality, tolerance of treatment, quality of life, and healthcare use (e.g. general practitioners visits, number and length of hospital stay) (Martyn et al. 1998, Löser C. 2010). Weight loss during an illness is a red flag for even the most inexperienced clinician, but malnutrition in the absence of manifest illness is rarely recognized as a modifiable risk factor for development of chronic diseases. The social determinants influencing food intake and hence malnutrition, e.g. loneliness and isolation, poverty, poorly fitting dentures, inaccessible food outlets, difficulty in cooking, or in supporting oneself to eat and drink, may be in operation long before associated comorbidities appear. Moreover, treatment and amelioration of these factors may delay or even prevent the onset of disease (Pryke et al. 2013).
For such a widespread significant problem, malnutrition has so far attracted very little attention in primary care. One factor of this phenomenon may be nutrition’s notable absence from most medical school curricula and postgraduate training, resulting in poor awareness, large knowledge gaps, and a deficit of nutrition-related competencies. Lack of ownership among clinicians is another factor, because malnutrition, like other processes such as pain, inflammation, and obesity, cuts across traditional clinical specialty boundaries instead of falling neatly within one or other. Reflecting this collective uncertainty and patchy knowledge, a host of unhelpful nutritional myths have also propagated and stabilized within our culture and have normalized nutritional problems. It would be timely to debunk the perception that weight loss is an inevitable part of ageing or that lower energy “healthy foods” are appropriate for everyone (Pryke et al. 2013).
There is ongoing debate in the literature about the merits of oral nutritional supplements (ONS) compared to first-line dietary advice (“food first”: information on food fortification, snacks, food choices). Skepticism regarding ONS relate to its largely hospital-focused evidence base. Issues around palatability, taste fatigue (particularly in the chronically ill requiring long-term supplementation), patient preference for “normal food”, and psychological factors were regarded as factors that might all impact the effectiveness of therapy. Thus, skeptics suggest to prefer dietary advice versus ONS. Conversely, superficial dietary advice runs the risk of at-risk patients simply increasing calories without addressing essential protein and micronutrient requirements, and it is unfeasible for a “food first” approach to address nutritional deficits in some patients, particularly those with anorexia and/or early satiety. Although dietary fortification and counselling can improve nutritional intake, the evidence base is weak for improved outcomes relative to the evidence for ONS, questioning whether “food first” can replicate the combination of nutrients found in ONS (Pryke et al. 2013). These results indicate that compared to dietary counseling, the use of ONS is based on more evidence in the treatment and prevention of malnutrition. Numerous clinical studies investigated the effect of nutritional therapy on disease-related malnutrition. Meta-analyses on treatment of disease-related malnutrition with medical nutrition show a reduction in complications and mortality, improvement of wound healing, and increase of quality of life (Elia et al. 2021, Elia et al. 2005, Stratton RJ. 2005).
Malnutrition in chronic obstructive pulmonary disease
Many COPD patients are in a state of marked undernutrition called pulmonary cachexia (Itoh et al. 2013). In total, 25-40% of all COPD patients exhibit weight loss, with 35% showing a reduced fat free mass index (Itoh et al. 2013). Malnourished COPD patients have a significantly higher 1-year mortality and are hospitalized more frequently (Hoong et al. 2017).
Many different factors contribute to nutritional abnormalities and muscle dysfunction in chronic respiratory diseases. Nutritional abnormalities are frequent in different chronic respiratory diseases such as COPD, bronchiectasis, cystic fibrosis (CF), interstitial fibrosis and lung cancer, and have important clinical consequences. However, nutritional abnormalities often remain underdiagnosed due to the relative lack of awareness of health professionals. Therefore, systematic anthropometry or even better, assessment of body composition, should be performed in all patients with chronic respiratory conditions, especially following exacerbation periods when malnutrition becomes more accentuated (Gea et al. 2018).
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung and total body disorder characterized by a progressive, persistent airflow obstruction. Most patients with COPD are lean, and often show decreased body weight. Many patients are in a state of marked undernutrition called pulmonary cachexia. Typical metabolic changes associated with the development of cachexia are an increased release of pro-inflammatory cytokines, as well as an overactivity of sympathetic nervous system, as indicated by the increased plasma concentrations of catecholamines. Both pro-inflammatory cytokines and catecholamines promote catabolic processes leading to skeletal muscle and fat mass wasting, such as stimulation of lipid utilization and skeletal muscle protein breakdown while decreasing energy intake and increasing energy expenditure (Müller et al. 2010).
In total, 25-40% of all COPD patients exhibit weight loss, with 25% of the patients having moderate to severe disease and 35% showing a severe disease with a reduced fat free mass index (Itoh et al. 2013). In a large United States (US) national outpatient trial 24% of 779 male COPD patients had a body weight <90% of ideal body weight. In The Netherlands, 35% of 255 consecutive patients admitted for intensive pulmonary rehabilitation weighed <90% of ideal body weight. In the UK, 36% of 69 outpatients with moderate to severe COPD weighed <90% of ideal body weight (Congleton J. 1999).
Decreased body weight has been identified as a factor for poor prognosis in patients with COPD. COPD patients with a BMI <20 kg/m2 have been shown to have a higher risk of acute exacerbations than patients with a BMI ≥20 kg/m2, and that patients exhibiting weight loss during a 1-year observation period are more prone to acute exacerbations than those without weight loss. A negative correlation between the BMI and duration of hospitalization has also been demonstrated. Furthermore, body weight is positively correlated to exercise tolerance and diffusing capacity of the lung (Itoh et al. 2013).
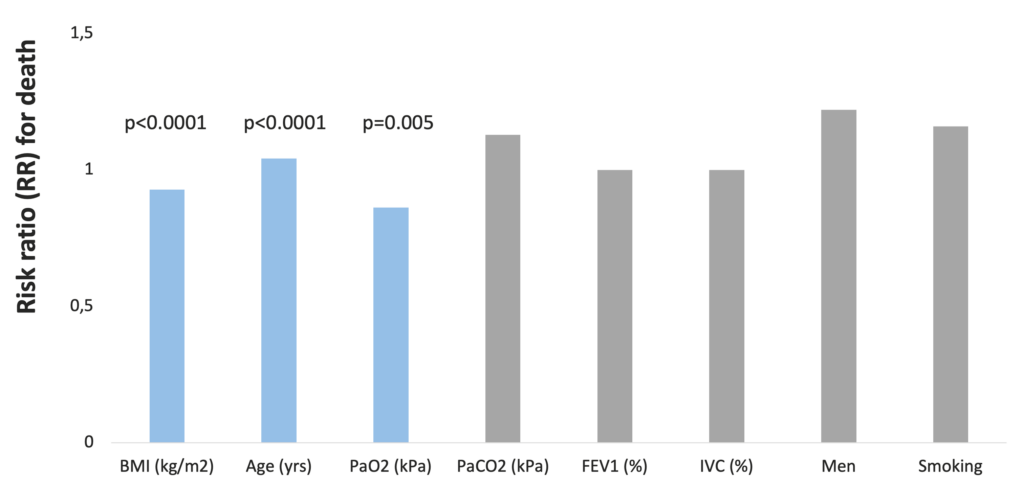
A retrospective study of 400 COPD patients revealed that low BMI (p<0.001), age (p<0.0001), and low arterial O2 tension (p<0.05) were significant independent predictors of increased mortality. After stratification of the group according to BMI, a threshold value of 25 kg/m2 was identified below which the mortality risk was clearly increased (Schols et al. 1998).
BMI is an independent predictor of mortality in COPD patients (Schols et al. 1998)
In a prospective cohort observational pilot study of 834 COPD outpatients in Australia, malnourished patients had a significantly higher 1-year mortality (27.7% vs. 12.1%; <0.001) and were hospitalized more frequently (1.11 SD 1.24 vs. 1.51 SD 1.43; p<0.051). Only malnutrition (OR=0.36, 95% CI 0.14-0.91; p<0.032), and emergency hospitalization rate (OR=1.58 95% CI 1.2-2.1; p<0.001) were independently associated with 1-year mortality. Length of hospital stay was almost twice the duration in those coded for malnutrition (11.57 SD±10.93 days vs. 6.67 SD±10.2 days; p<0.003) and at almost double the cost (AUD $23,652 SD±$26,472 vs. $12,362 SD±$21,865; p<0.002) than those who were well-nourished (Hoong et al. 2017).
Clinical nutrition in COPD
Weight gain (>2 kg/8 weeks) in patients with COPD is a significant predictor of survival (Schols et al. 1998). Casein feeding has been shown to induce higher net protein sythesis due to lower whole-body protein breakdown in COPD patients (Engelen et al. 2012).
Cachexia is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass. The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders). From an energy point of view, an increased weight loss may be related to a decrease in thermodynamic efficiency. Variable thermodynamic efficiency in metabolic systems is related not only to differences in weight but also may confer metabolic advantages or drawbacks. At present, it is widely held that elevated resting energy expenditure (REE) is a major determinant in the development of malnutrition in cachectic patients. Resting energy metabolism represents the combustion of fuel sources needed to provide energy for metabolic processes involved in maintaining the function and integrity of cells and body organs and for the mechanical processes involved in keeping the body alive. It is appropriate, therefore, to presume that abnormalities in carbohydrate, lipid, and protein metabolism are major biochemical bases of elevated REE (Hyltander et al. 1991, Argiles et al. 2014).
Treatment for cachexia has concentrated on increasing food intake, although that alone is unable to reverse the metabolic changes (Tisdale et al. 2004). Due to the hypercatabolic state and increased protein breakdown seen in malnourished / cachectic patients, increased energy and protein intake is vital in slowing down / stabilizing or even reversing weight loss and lean body mass /muscle wasting in cachectic patients (Cawood et al. 2012, Broekhuizen et al. 2005a, Guagnozzi et al. 2012).
By population prevalence, one of the most frequent cachexia subtypes is COPD cachexia (von Haehling & Anker. 2010). In a cross-sectional study of 99 patients with COPD, fat mass, leptin, and pseudouridine – a stable urinary end product of RNA turnover and hence a marker of cellular protein breakdown – were significantly different between non-cachectic and cachectic patients (p<0.001). Non-cachectic patients had a body compositional shift toward a lower fat-free mass and a higher fat mass compared to healthy controls (Broekhuizen et al. 2005a).
In a prospective study of 203 COPD patients, weight gain (>2 kg/8 weeks) in depleted and non-depleted patients with COPD, as well as increase in maximal inspiratory mouth pressure during the 8-week treatment, were significant predictors of survival (Schols et al. 1998).
Several recent publications indicate that the maximum stimulation of muscle protein fractional synthetic rate occurs with intake of 20-30 g protein. This finding has led to the concept that there is a maximal anabolic response to protein intake with a meal, and that the normal amount of protein eaten with dinner will generally exceed the maximally effective intake of protein. However, protein breakdown has not been taken into account when evaluating the anabolic response to protein intake. Protein anabolism occurs only when protein synthesis exceeds protein breakdown. Higher protein intakes when protein synthesis is maximized is characterized by suppressed protein breakdown and via that mechanism leads to a greater anabolic response. This explains why when net protein synthesis is measured, the relationship between amino acid availability and net gain remains linear, without any apparent plateau of effect at higher levels of availability. It may be concluded that there is no practical upper limit to the anabolic response to protein or amino acid intake in the context of a meal (Deutz et al. 2013).
In a study of 12 COPD patients, intake of a 15 g hydrolyzed casein or hydrolyzed whey protein meal with or without leucine co-ingestion was investigated. Whole-body protein synthesis was greater after intake of the hydrolyzed casein protein meal (p<0.05) whereas the hydrolyzed whey protein meal reduced whole-body protein breakdown more (p<0.01). Net protein synthesis was stimulated comparably, with a protein conversion rate greater than 70%. Addition of leucine did not modify the insulin, whole-body protein breakdown, whole-body protein synthesis, or myofibrillar protein breakdown response. The authors concluded that hydrolyzed casein and whey protein meals comparably and efficiently stimulate whole-body protein anabolism in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion (Jonker et al. 2014). In a study of 8 COPD patients and 8 healthy subjects, net protein synthesis was higher during casein feeding in COPD due to lower whole-body protein breakdown (p<0.05). Higher splanchnic amino acid extraction was found during exercise during casein and whey feeding in COPD (p<0.05). Lactate levels during exercise were higher in COPD (p<0.05) independent of the protein. Post-exercise, lower net protein synthesis values were found independent of protein type in both groups. Casein resulted in more protein anabolism than whey protein which was maintained during and following exercise in COPD (Engelen et al. 2012).
High calorie intake, especially in the form of carbohydrates, increases CO2 production and may precipitate respiratory failure in patients with severe lung disease. Energy obtained from fat results in less CO2 and thus may permit a reduced level of alveolar ventilation for any given arterial CO2 tension (Efthimiou et al. 1992). In patients with COPD, low-carbohydrate-high-fat nutrition is more beneficial (Kuo et al. 1993, Frankofort et al. 1991, Cai et al. 2003, Angelillo et al. 1985).
Though increased energy intake is very important in treating malnutrition / cachexia of patients with chronic diseases, the source of this extra energy also plays an important role in the therapy of the primary disease. Due to the hypercatabolic state and increased muscle wasting, a higher energy and protein intake may be beneficial for COPD patients. High calorie intake, especially in the form of carbohydrates, increases carbon-dioxide (CO2) production and may precipitate respiratory failure in patients with severe lung disease. Energy obtained from fat results in less CO2 and thus may permit a reduced level of alveolar ventilation for any given arterial CO2 tension (Efthimiou et al. 1992). Pulmonary rehabilitation, associating physical exercise and nutritional supplements containing ω-3 polyunsaturated fatty acids, for at least three months, is the reference therapy of undernourished COPD patients (Samaras et al. 2014).
In a study of 10 patients with severe stable COPD, consumption of a carbohydrate-rich drink resulted in significantly greater increases of minute ventilation, CO2 level, oxygen (O2) consumption, respiratory quotient, arterial CO2 tension, and a greater fall in the distance walked in 6 minutes, compared to the consumption of a fat-rich drink. The increase in the CO2 level correlated significantly with the decrease in the 6-minute walking distance (Efthimiou et al. 1992).
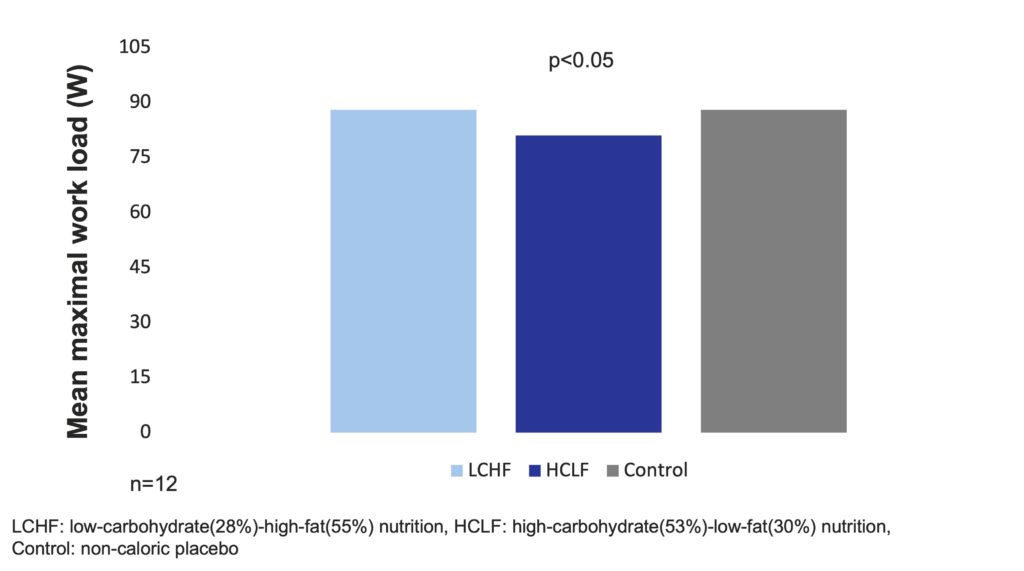
When isocaloric meals of low-carbohydrate (28%) and high-fat (55%) (LCHF) or high-carbohydrate (53%) and low-fat (30%) (HCLF) or a noncaloric placebo were given to 12 stable subjects with chronic airflow obstruction, mean maximal work load was significantly lower (p<0.05) in the HCLF (81±24 W) than in the LCHF group (88±21 W) or in the control group (88±24 W). The authors concluded that meals with a lower carbohydrate and higher fat content may less likely impair work performance of patients with chronic airflow obstruction in the absorptive phase than meals with a higher carbohydrate and lower fat content (Frankofort et al. 1991).
Low-carbohydrate-high-fat nutrition does not significantly decrease mean maximal work load in COPD patients (Frankofort et al. 1991)
In a study of 12 clinically stable, ambulatory patients with COPD, compared to the high-fat diet, the high-carbohydrate diet led to significantly higher production of CO2, minute ventilation, O2 consumption, and respiratory quotient 30-60 minutes after the consumption, and the differences could last for about 1.5 hour. According to these results, a high-fat diet is more beneficial for the COPD patients than a high-carbohydrate diet (Kuo et al. 1993).
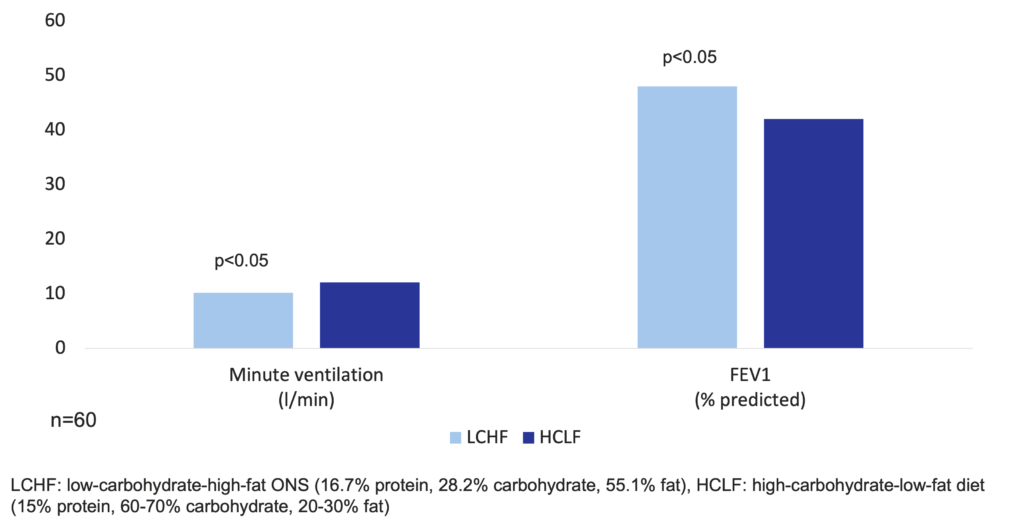
In a study of 60 COPD patients with low body weight (<90% ideal body weight), a LCHF ONS (16.7% protein, 55.1% fat, and 28.2% carbohydrate, 2-3 cans of 237 ml daily) in the evening as part of the diet compared to the control diet (dietary counseling for a high-carbohydrate diet of 15% protein, 20-30% fat, and 60-70% carbohydrate) significantly increased forced expiratory volume in 1 second (FEV1) (p<0.05) (Cai et al. 2003).
Pulmonary function in COPD patients improves with a low-carbohydrate-high-fat ONS as compared with the traditional high-carbohydrate-low-fat diet (Cai et al. 2003)
In a randomized, double-blinded study, 12 patients with COPD and hypercapnia were fed low, moderate, and high carbohydrate diets to determine the effect on metabolic and ventilatory values. The LCHF nutrition consisted of 28% carbohydrate calories and 55% fat calories and resulted in significantly lower production of CO2 (p<0.002), respiratory quotient (p<0.001), and arterial pCO2 (p<0.05). At the end of the 15-day study, both the forced vital capacity (p<0.05) and the FEV1 (p<0.05) improved by 22% over baseline values (Angelillo et al. 1985).
Low-carbohydrate-high-fat nutrition significantly increases respiratory parameters in COPD patients (Angelillo et al. 1985)
Safety of a low carbohydrate diet
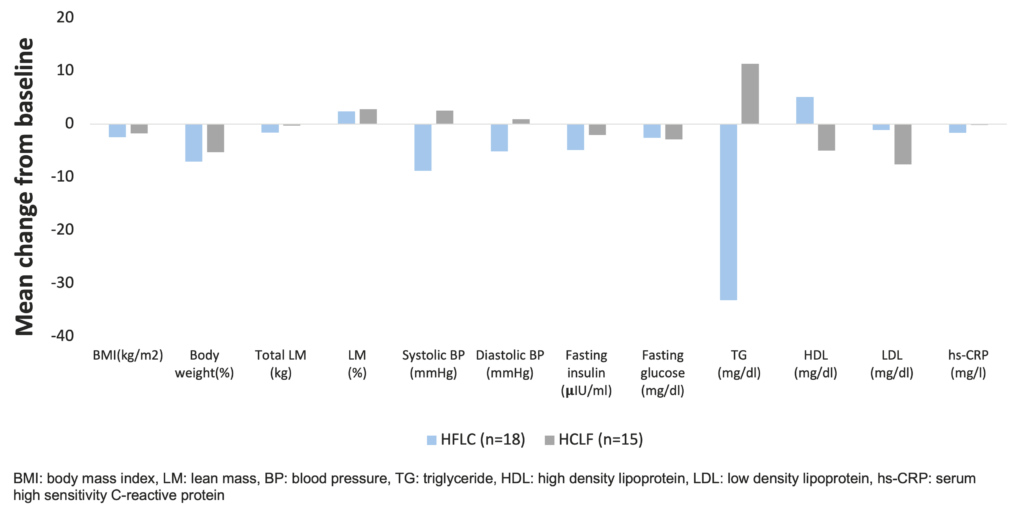
Larosa et al. were the first to demonstrate in 1980 (and confirmed by others many times since) that low-carbohydrate diets (30-130 g/day) do not necessarily require higher fat or protein intakes, and a spontaneous decrease in overall calorie consumption frequently results in little protein or fat added back for the carbohydrate removed (Larosa et al. 1980). This causes the phenomenon that a very low carbohydrate diet ameliorates all the investigated anthropometric laboratory parameters (BMI, abdominal fat, triglycerides, HDL, triglycerides/HDL ratio, ApoB/ApoA1 ratio, small LDL, blood glucose, plasma insulin, saturated fatty acid) of patients with atherogenic dyslipidemia (Hite et al. 2011). In a study, which randomized obese subjects (29.0-44.6 kg/m2) recruited from Boston Medical Center to a hypocaloric low-fat-high-carbohydrate / LFHC (n=26) or high-fat-low-carbohydrate / HFLC (n=29) diet for 12 weeks, the HFLC group had greater improvements in blood lipids and systemic inflammation with similar changes in body weight and composition, relative to the LFHC group (Ruth et al. 2013).
Beneficial effects of a low-carbohydrate diet in obese subjects (Ruth et al. 2013)
In a large, epidemiological cohort study of individuals aged 35–70 years in 18 countries with a median follow-up of 7.4 years (IQR 5.3-9.3), dietary intake of 135,335 individuals was recorded using validated food frequency questionnaires. Participants were categorized into quintiles of nutrient intake (carbohydrate, fats, and protein) based on percentage of energy provided by nutrients. During follow-up, 5,796 deaths and 4,784 major cardiovascular disease events were documented. Higher carbohydrate intake was associated with an increased risk of total mortality (highest [quintile 5] vs lowest quintile [quintile 1] category, HR=1.28 [95% CI 1.12-1.46], ptrend=0.0001) but not with the risk of cardiovascular disease or cardiovascular disease mortality. Intake of total fat and each type of fat was associated with lower risk of total mortality (quintile 5 vs quintile 1, total fat: HR=0.77 [95% CI 0.67-0.87], ptrend<0.0001; saturated fat [SF], HR=0.86 [0.76-0.99], ptrend=0.0088; monounsaturated fat [MUFA]: HR=0.81 [95% CI 0.71-0.92], ptrend<0.0001; and polyunsaturated fat [PUFA]: HR=0.80 [95% CI 0.71-0.89], ptrend<0.0001). Higher saturated fat intake was associated with lower risk of stroke (quintile 5 vs quintile 1, HR=0.79 [95% CI 0.64-0.98], ptrend=0.0498). Total fat and saturated and unsaturated fats were not significantly associated with risk of myocardial infarction or cardiovascular disease mortality. Based on these results, the authors suggest the reconsideration of global dietary guidelines (Dehghan et al. 2017).
In a large, epidemiological cohort study, high carbohydrate intake was associated with higher risk of total mortality, whereas total fat and individual types of fat were related to lower total mortality (Dehghan et al. 2017).
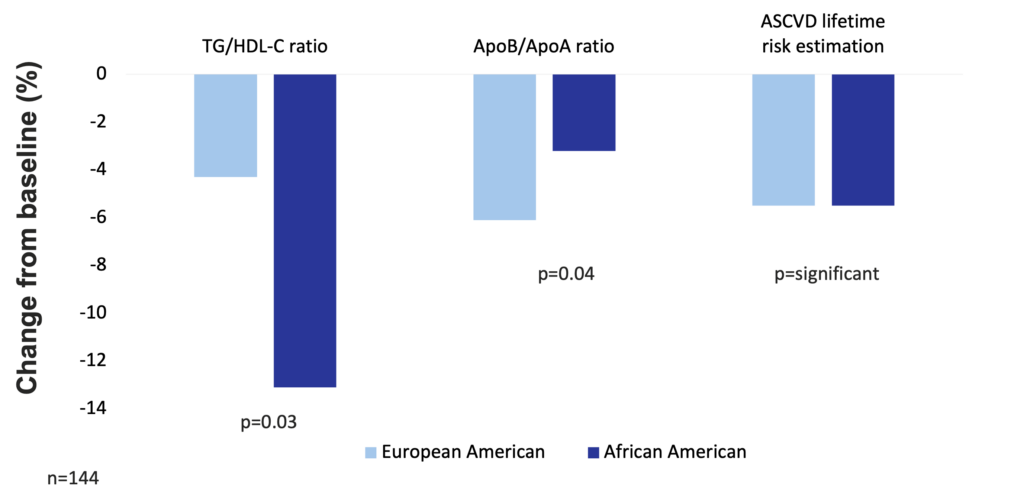
One hundred and forty-four premenopausal women of age 21-50 years with class I/II obesity (BMI 30-39.9 kg/m2) were recruited in a study to keep a balanced high-fat diet (50% fat, 30% carbohydrate, 15% protein, with a balanced fat content – 1/3 saturated fatty acids, 1/3 monounsaturated fatty acids, 1/3 polyunsaturated fatty acids) for 16 weeks. To control the effects of high simple carbohydrate intake, total carbohydrate was maintained as 50% sugars (mono- and disaccharides) and 50% starches. Results in European American and African American participants were analyzed separately. Consuming the balanced high-fat diet significantly reduced cardiovascular risk by 5.5% (increased HDL particle size, increased the number of large HDL particle size, and increased apolipoprotein AI level) in both groups. In addition, European American women had significant reductions in fasting insulin levels (by 24.8%) and in HOMA-insulin resistance (by 29%). In the group of European American women, the most significant improvements occurred in VLDL particle size, apolipoprotein B levels, serum triglyceride, number of plasma LDL particles, and serum LDL cholesterol (Niswender et al. 2018).
High-fat balanced diet improves atherosclerotic cardiovascular disease risk in obese premenopausal women (Niswender et al. 2018)
ω-3 fatty acids supplementation decreases inflammation in patients with COPD (Fulton et al. 2013). ω-3 fatty acids decrease the number of exacerbations, drug consumption, and improves 6-minute walking distance (6MWD), exercise-induced fatigue, cycle endurance time, and dyspnoea in COPD patients (Fekete et al. 2021, van de Bool et al. 2017, Calder et al. 2018).
Cachectic and non-cachectic COPD patients have a greater systemic inflammatory response (p<0.05) than do healthy controls, as reflected in C-reactive protein (CRP), soluble tumor necrosis factor receptor (TNF-R)75 and interleukin (IL)-6 concentrations (Broekhuizen et al. 2005b). Two multicentric randomized clinical trials have shown that the association of androgen therapy with physical exercise and ONS containing ω-3 polyunsaturated fatty acids, for at least 3 months, is associated with an improved clinical outcome and survival (Samaras et al. 2014).
According to the proposed cascade between long-chain ω-3 PUFA supplementation and the outcome measures, ω-3 PUFA supplementation increases the ω-3 fatty acid content of the erythrocytes, which decreases the intensity of chronic inflammation (measured by the levels of inflammatory biomarkers) and thus the frequency and severity of exacerbations. Moreover, decreasing the level of chronic inflammation ameliorates the small airway functions, decrease the work of breathing and dyspnoea, thus increases the functional exercise capacity (measurable by the 6-min walking distance / 6MWD) and in the end, ameliorates patient’s well-being measurable by the Hospital Anxiety and Depression Scale (HADS) and the Chronic Respiratory Disease Questionnaire (CRQ) (Fulton et al. 2013).
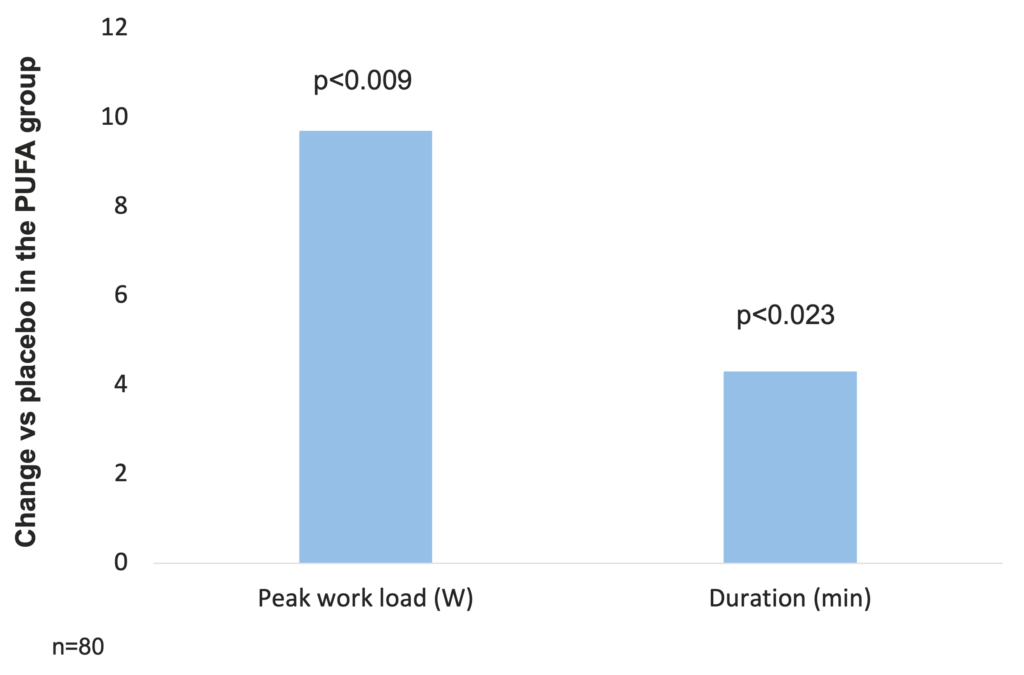
In a study investigating the effect of PUFA modulation on systemic inflammation, reversal of muscle wasting, and functional status in COPD, 80 patients with COPD (of which 57 men) with FEV1 37.3 (13.8) % predicted received 9 g PUFA or placebo daily in a double blind randomized fashion during an 8 week rehabilitation program. Both groups had similar increases in weight, fat-free mass (FFM), and muscle strength. The peak load of the incremental exercise test increased more in the PUFA group than in the placebo group (difference in increase 9.7 W (95% CI 2.5 to 17.0), p=0.009) even after adjustment for FFM. The duration of the constant work rate test also increased more in patients receiving PUFA (difference in increase 4.3 min (95% CI 0.6 to 7.9), p=0.023) (Broekhuizen et al. 2005b).
PUFA supplementation increases peak load of incremental exercise test and exercise duration in COPD patients (Broekhuizen et al. 2005b)
Observational data support the hypothesis that ω-3 PUFA may provide a therapeutic strategy for managing COPD. (Wood LG. 2015).
In a study of 404 COPD patients older than 40 years of age, 4.7% (n=19) of patients took ω-3 fatty acids regularly, mainly on the recommendation of their physicians, at the recommended daily dose (0.25-0.50 g/day). Compared to their peers, these patients had fewer comorbidities (hypertension: 52.6% vs. 72.1%, p=0.066), consumed fewer drugs (short-acting bronchodilators: 25.3% vs. 51.7%, p=0.031), had fewer exacerbations (1 [1-3] vs. 2 [1-4]; p=0.029), and higher 6MWD (300 m [177-387] vs. 251 m [150-345], p=0.121) (Fekete et al. 2021).
In 81 COPD patients with low muscle mass, an ONS enriched with leucine, vitamin D, and ω-3 fatty acids, and placebo treatment both improved skeletal muscle mass (+0.4 ± 0.1 kg, p<0.001), quadriceps muscle strength (+12.3 ± 2.3 Nm, p<0.001), and cycle endurance time (+191.4 ± 34.3 s, p<0.001). Inspiratory muscle strength only improved in the ONS (+0.5 ± 0.1 kPa, p=0.001) group, while steps/day declined in the placebo group (-18%, p=0.005) (van de Bool et al. 2017).
In a clinical study, 45 COPD patients aged ≥50 years with moderate-to-severe COPD and involuntary weight loss or low body mass index (16–18 kg/m2) were randomized in a 1:1 ratio to receive targeted medical nutrition (TMN: ~230 kcal; 2 g ω-3 fatty acids; 10 μg 25-hydroxy-vitamin D3 [25-OHD]) or isocaloric comparator twice daily for 12 weeks. Both groups gained weight, but the TMN group gained comparatively more fat mass (p=0.0013). Reductions in systolic blood pressure (p=0.0418) and secondary endpoints of triglycerides (p=0.0217) and exercise-induced fatigue (p=0.0223) and dyspnoea (p=0.0382), and increases in high-density lipoprotein cholesterol (p=0.0254), were observed in the TMN vs. the comparator group by week 12 (Calder et al. 2018).
Targeted medical nutrition has beneficial effects on tiredness and dyspnoea caused by physical exertion in COPD (Calder et al. 2018)
High-dose vitamin D treatment during respiratory rehabilitation leads to a marked improvement of the inspiratory muscle strength and maximum O2 intake in COPD patients (Hornikx et al. 2012). FEV1 has been well maintained in patients with higher intakes of vitamin C and/or vitamin E (Itoh et al. 2013)
In children, micronutrient malnutrition is a cause of stunting and may be accompanied by metabolic adaptations in response to nutrient deficiencies that increase the risk of later obesity and related chronic disease. In adults, deficiencies in key antioxidant micronutrients may promote oxidative stress, creating an environment in which the incidence and severity of diseases such as diabetes, CVD, and cancer are increased. Folate deficiency in adults may increase serum levels of homocysteine, an established risk factor for heart disease (Eckhardt CL. 2006).
A study with highly precise measurement of the vitamin D concentration in blood samples of 433 COPD patients and 325 healthy subjects showed that vitamin D deficiency was more common among COPD patients than among healthy subjects, and that the FEV1 and blood level of vitamin D were positively correlated in COPD patients. COPD patients had an increased risk for vitamin D deficiency compared to controls after adjustment for seasonality, age, smoking, and BMI (Perssons et al. 2012). Another study of 414 COPD patients found that 25-OHD levels correlated significantly with FEV1 (r=0.28, p<0.0001). Compared with 31% of the smokers with normal lung function, as many as 60% and 77% of patients with Global Initiative for Obstructive Lung Disease (GOLD) stage 3 and 4 exhibited deficient 25-OHD levels of <20 ng/ml (p<0.0001). From GOLD stage 2 onwards, 25-OHD levels differed significantly from those of healthy smokers (20.35, 18.8, and 16.0 ng/ml for GOLD 2, 3 and 4, respectively; [p<0.0001]). Mean 25-OHD levels were almost 33% lower in patients with COPD with GOLD 4 compared to healthy smokers (Janssens et al. 2010).
In a randomized controlled trial of high-dose vitamin D treatment during respiratory rehabilitation in 50 COPD patients, a more marked improvement of the inspiratory muscle strength and maximum O2 intake was noted in the group of patients undergoing rehabilitation while receiving 100,000 IU of vitamin D at monthly intervals for 3 months, as compared to the placebo group (Hornikx et al. 2012).
Several studies have examined the effects of exogenous vitamin C or E supplementation on oxidative stress in COPD patients. In a study of 35 COPD patients, investigation of the effects of placebo, 400 mg/day vitamin E, 200 mg/day vitamin E or 250 mg/day vitamin C for 12 weeks showed that vitamin E or C supplementation did not change the level of endogenous DNA damage of the white blood cells, but significantly suppressed H2O2-induced DNA breakage (Wu et al. 2007).
In a single-blind uncontrolled comparative clinical study of 45 COPD patients, adding 500 mg ascorbic acid twice daily to the treatment with salbutamol 100 μg and beclomethasone 50 μg twice daily delayed the lung function deterioration (FEV1/FVC ratio) when compared to the treatment of salbutamol 100 μg and beclomethasone 50 μg twice daily only (Ansari MA. 2010).
Another study in 30 COPD patients treated with 400 IU vitamin E for 12 weeks demonstrated significant reductions in the plasma malondialdehyde (MDA) levels, although neither the plasma 𝛂-tocopherol levels and red blood cell superoxide dismutase levels, nor the lung function showed any significant changes after the vitamin E supplementation (Daga et al. 2003). With regards to the relationship of the intakes of vitamins C and E to the pulmonary function, most cross-sectional studies in healthy subjects and COPD patients reported so far have shown that FEV1 is well maintained in individuals with higher intakes of vitamin C and/or vitamin E (Itoh et al. 2013).
A large-scale randomized controlled trial of 38,597 participants (Women’s Health Study) demonstrated that the risk of development of chronic lung diseases, such as COPD, during a 10-year observation period was reduced by 10% in a group of subjects taking vitamin E (600 IU) on alternate days (Agler et al. 2011). Meanwhile, the interactions between vitamins and antioxidant enzymes were also investigated. For example, it was reported that deficient vitamin C intake resulted in a marked depression of FEV1 in heavy tobacco smokers bearing a mutant gene encoding GCLC, a subunit of glutamate-cysteine ligase involved in glutathione synthesis (Siedlinski et al. 2008). Vitamin D deficiency is thought to be a risk factor for concurrent osteoporosis (Graat-Verboom et al. 2012). Vitamin D is known to have effects on immune reactions, inflammation, remodeling of airways, and muscle strength (Itoh et al. 2013). It has been inferred that the limited hours of sunbathing in severe COPD patients may be responsible for the low blood vitamin D levels in these patients (Kunisaki et al. 2011).
L-carnitine supplementation with respiratory muscle training improves physical performance and inspiratory muscle strength in COPD patients (Borghi-Silva A. et al. 2006).
4-N-trimethylammonium-3-hydroxybutyric acid (L-carnitine), an amino acid derivative, is an endogenous mitochondrial membrane compound (Yu et al. 2011) with antioxidant effects and is found to be a protective agent against many diseases (El-Ashmawy et al. 2014). L-carnitine promotes lipid combustion through its effect of mediating fatty acid transport into the mitochondria, consequently effecting increased energy production (Silverio et al. 2011).
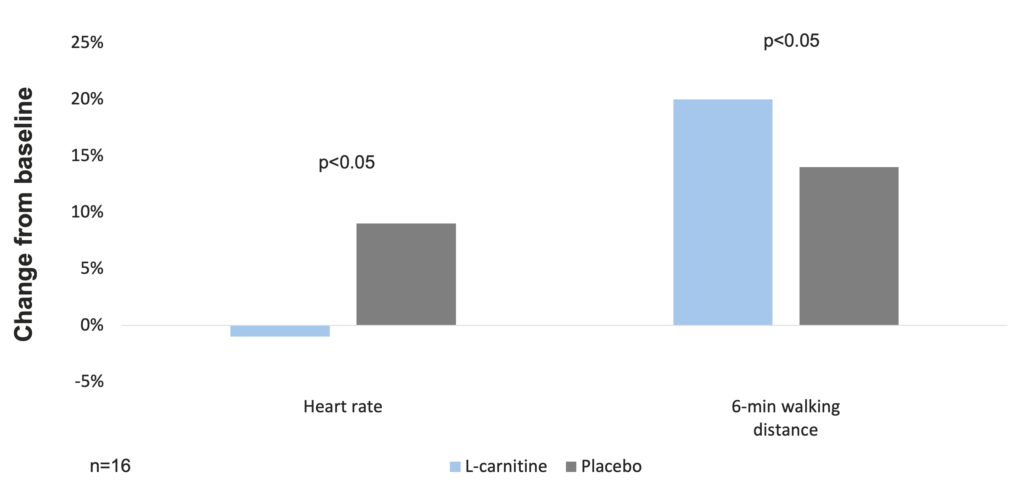
In a randomized controlled trial of 6-week respiratory rehabilitation with concurrent administration of L-carnitine (2 g/day, per os) or placebo in 16 COPD patients, blood lactate, blood pressure, O2 saturation, and heart rate at identical exercise levels were lower in the L-carnitine group after training compared to the placebo group (p<0.05). Inspiratory muscle strength and walking test tolerance were significantly improved in both groups, but the gains of L-carnitine group were significantly higher than those of the placebo-treated group (40±14 vs 14±5 cm H2O, and 87±30 vs 34±29 m, respectively; p<0.05). Blood lactate concentration was significantly lower in the treated patients than in the placebo group (1.6±0.7 vs 2.3±0.7 mM, p<0.05). The present data suggest that L-carnitine can improve exercise tolerance and inspiratory muscle strength in COPD patients, as well as reduce lactate production (Borghi-Silva A. et al. 2006).
L-carnitine supplementation with respiratory muscle training improves physical performance in COPD patients (Borghi-Silva A. et al. 2006)
MediDrink Pulmo
MediDrink Pulmo is a nutritionally complete, 2.0 kcal/ml, gluten- and lactose-free, ready-to-consume food for special medical purposes (as per the EU regulation 128/2016) for the dietary management of malnutrition in patients with compromised respiratory functions. MediDrink Pulmo is suitable as supplemental nutrition or as the sole source of nutrition.
MediDrink Pulmo is not suitable for children below 3 years of age, patients suffering from galactosemia, and patients with hereditary fructose intolerance/fructosemia.
MediDrink Pulmo is available in 3 different flavors: chocolate, strawberry, and vanilla.
Compliance, safety, tolerability
Compliance is significantly greater to ≥2 kcal/ml ONS compared to standard 1.5 kcal/ml ones (Hubbard et al. 2012). Overall pleasantness of taste for patients is significantly better for milk-based supplements (Darmon et al. 2008).
MediDrink Pulmo is suitable also for diabetic COPD patients (Medifood Data on file 2020).
Compliance
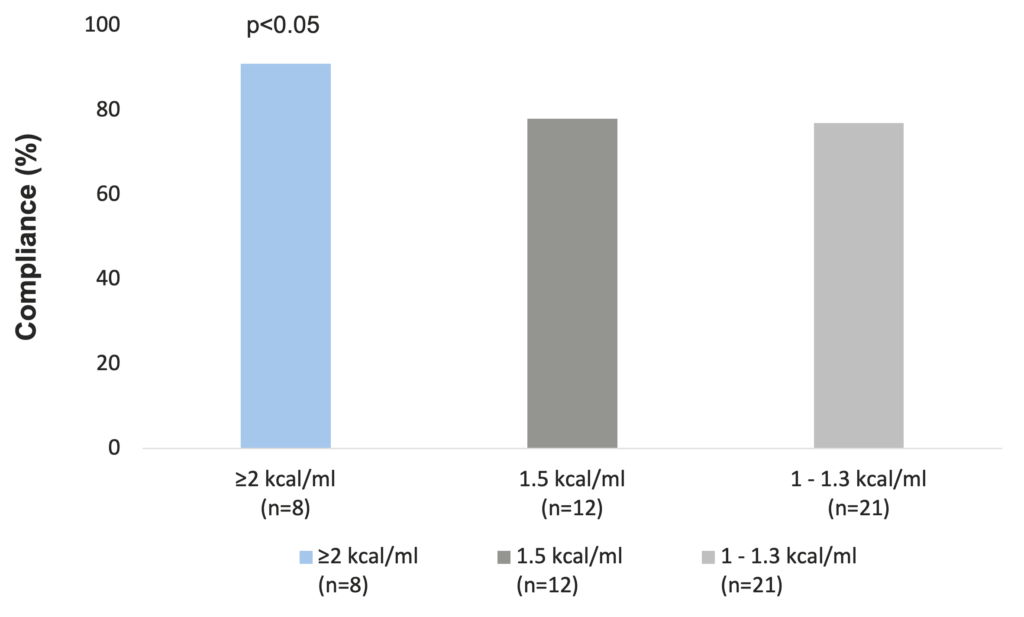
According to data from a meta-analysis, the overall compliance to ONS is good, especially with higher energy-density ONS, resulting in improvements in patients’ total energy intakes that have been linked to clinical benefits. A range of ONS-related attributes have been associated with compliance. Recent comparisons suggest significantly greater compliance and energy intakes with the use of small volume, energy dense products (energy content ≥2 kcal/ml) compared to standard 1.5 kcal/ml ONS. Therefore, in patients who struggle to ingest the prescribed volume of ONS, a change to a higher energy density ONS could increase total energy intake and reduce wastage. This strategy could be employed in the nutritional management of malnourished individuals (Hubbard et al. 2012).
Compliance is greater with higher energy-density ONS (Hubbard et al. 2012)
A randomized controlled interventional trial of 77 elderly nursing home residents with high functional impairment showed that a low-volume, nutrient- and energy-dense ONS was well accepted and resulted in significant improvements of nutritional status and, thus, was effective to support treatment of malnutrition (Stange et al. 2013).
Taste
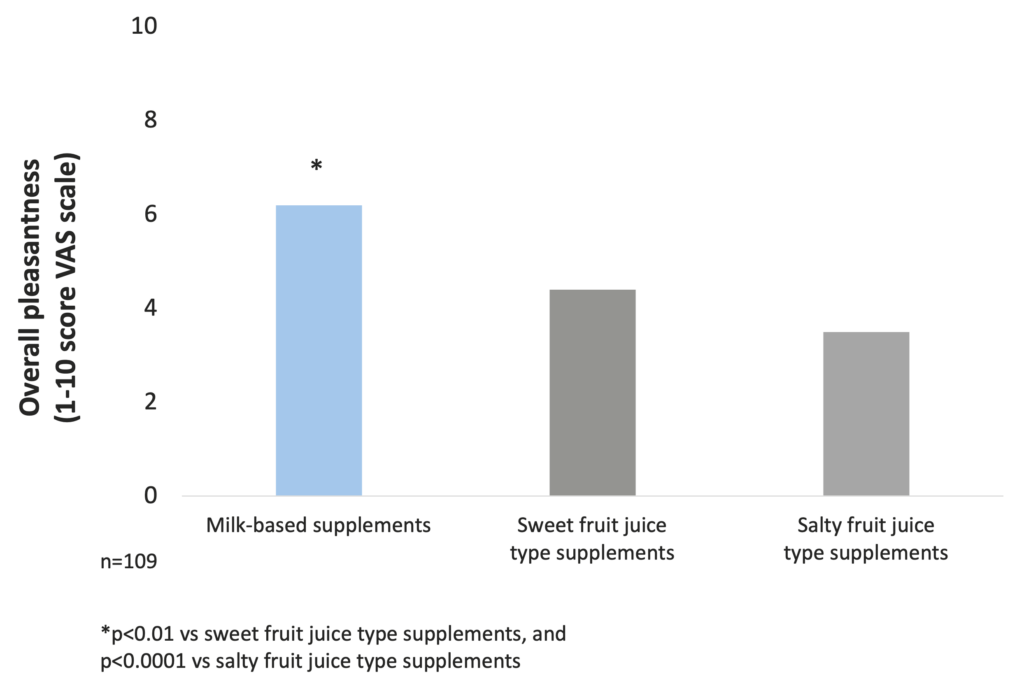
The investigation of taste preferences of milk-based and fruit juice-type supplements in malnourished in-patients showed that the overall pleasantness is significantly better for milk-based supplements than for sweet and salty fruit juice-type products (Darmon et al. 2008).
Pleasantness of milk-based ONS is significantly better than that of sweet and salty fruit juice-type products (Darmon et al. 2008)
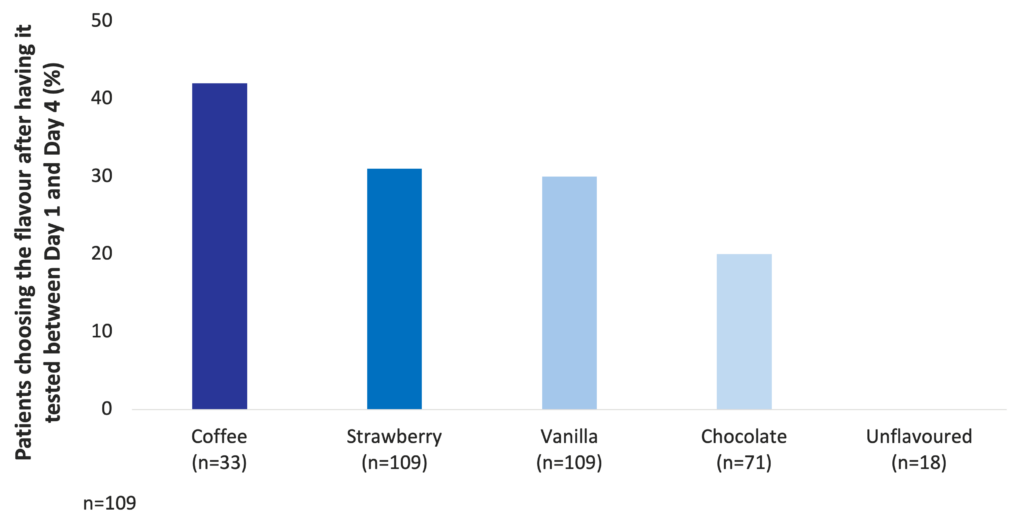
Among milk-based ONS, coffee, strawberry, vanilla, and chocolate are the most preferred flavours (Darmon et al. 2008).
Coffee, strawberry, vanilla, and chocolate are the most preferred flavours among milk-based ONS (Darmon et al. 2008)
Glycemic load
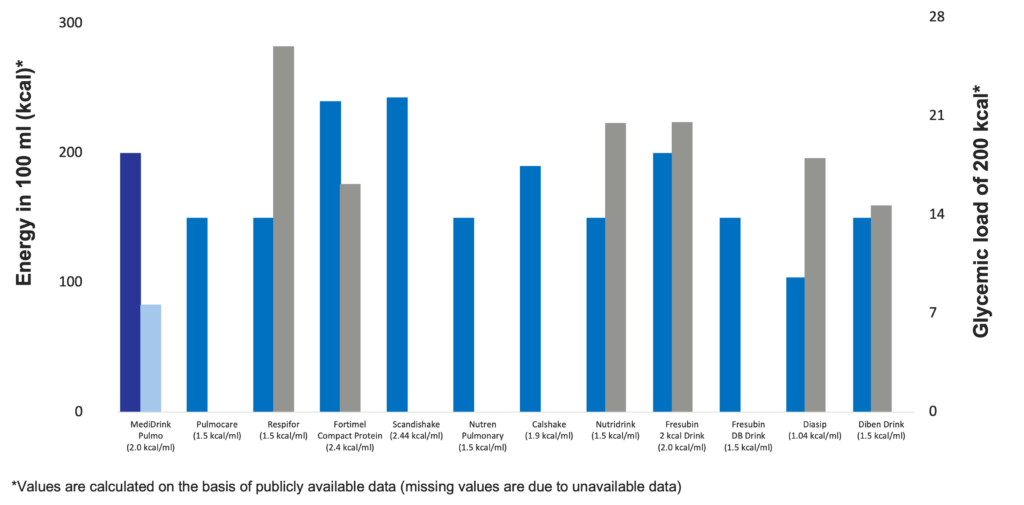
The concept of glycemic load (GL) has been introduced by Walter Willett at the Harvard School of Public Health in 2004. While glycemic index (GI) ranks the different sources of carbohydrates according to their blood sugar-raising capacity, GL takes also into account the quantity of food / carbohydrates consumed. GL is calculated by multiplying the GI of the carbohydrates by the quantity of the carbohydrates taken in. Therefore, the GL of a certain food depends not only on the GI but also the quantity consumed (Venn & Green 2007, Fajcsák & Lelovics. 2006). Based on the calculation of GL, MediDrink Pulmo has the lowest GL and therefore the smallest strain on the metabolism of the patients among the different ONS depicted below. Thus, MediDrink Pulmo is suitable also for diabetic patients, since when consuming the same amounts of kilocalories, the GL of MediDrink Pulmo is lower than that of the ONS specifically designed for the diabetic patients.
Energy content and glycemic load of several oral nutritional supplements
(Medifood data on file 2018, [Complete therapy with clinical nutrition] Nutricia 2016, https://www.abbottnutrition.ie/downloads/4.-Pulmocare-datasheet-Ireland-version-4-October-2019.pdf (accessed on 15th November 2023), https://www.direct.nutricia.it/prodotto/fortimel-compact-vaniglia-4x-confezione-125-ml (accessed on 15th November 2023), https://www.fresubin.be/fr-be/produits/fresubin-db-drink/ accessed on 7th November 2023, https://www.fresenius-kabi.com/nl/documents/Calshake®.pdf (accessed on 15th November 2018), https://www.fresenius-kabi.com/ie/documents/Calshake_Powder-d3ia8wRjwkkU7JuYJ7Ynfi1md03-BOSvSIqunUXr8x0.pdf (accessed on 15th November 2018), https://www.fresenius-kabi.com/nl/producten/calshake (accessed on 15th November 2018), https://www.nestlehealthscience.us/brands/nutren/nutren-pulmonary-hcp (accessed on 15th November 2018), https://www.nutricia.be/fr_be/produits/respifor.html#tabs-83e0f52aea-item-8691bfc754-tab (accessed on 15th November 2023), https://www.nutricia.nl/content/dam/sn/local/bnl/sn-hcp/website-assets/voedingstabellen/nl/sip-feed/Voedingstabel_Scandishake_Mix_032021.pdf (accessed on 9th November 2023)
References
[Complete therapy with clinical nutrition] – Nutricia, 15th June 2016
Eckhardt CL. Micronutrient malnutrition, obesity, and chronic disease in countries undergoing the nutrition transition: potential links and program/policy implications. 2006. International Food Policy Research Institute FCND Discussion Paper 213. (accessed on 12th November 2018)
Elia M. et al. To screen or not to screen for adult malnutrition? Clin Nutr 2005;24:867-84.
Fajcsák Zs. & Lelovics Zs. [Glycemic index and glycemic load]. Új Diéta 2006;4:28-9.
Freijer K. et al. The economic costs of disease related malnutrition. Clin Nutr 2013;32:136-41.
Hite AH. et al. Low-carbohydrate diet review: shifting the paradigm. Nutr Clin Pract 2011;26:300-8.
https://www.abbottnutrition.ie/downloads/4.-Pulmocare-datasheet-Ireland-version-4-October-2019.pdf (accessed on 15th November 2023)
https://www.direct.nutricia.it/prodotto/fortimel-compact-vaniglia-4x-confezione-125-ml (accessed on 15th November 2023)
https://www.fresubin.be/fr-be/produits/fresubin-db-drink/ accessed on 7th November 2023
https://www.fresenius-kabi.com/nl/documents/Calshake®.pdf (accessed on 15th November 2018)
https://www.fresenius-kabi.com/ie/documents/Calshake_Powder-d3ia8wRjwkkU7JuYJ7Ynfi1md03-BOSvSIqunUXr8x0.pdf (accessed on 15th November 2018)
https://www.fresenius-kabi.com/nl/producten/calshake (accessed on 15th November 2018)
https://www.nestlehealthscience.us/brands/nutren/nutren-pulmonary-hcp (accessed on 15th November 2018)
http://www.fresenius-kabi.co.uk/ (accessed on 15th November 2018)
https://www.nutricia.be/fr_be/produits/respifor.html#tabs-83e0f52aea-item-8691bfc754-tab (accessed on 15th November 2023)
https://www.nutricia.nl/content/dam/sn/local/bnl/sn-hcp/website-assets/voedingstabellen/nl/sip-feed/Voedingstabel_Scandishake_Mix_032021.pdf (accessed on 9th November 2023)
https://www.who.int/features/qa/malnutrition/en/ accessed on 23rd March 2020
Itoh M. et al. Undernutrition in patients with COPD and its treatment. Nutrients 2013;5:1316-35.
Ljungqvist O. et al. The European fight against malnutrition. Clin Nutr 2010;29:149-50.
Ljungqvist O. Man FD. Under nutrition: a major health problem in Europe. Nutr Hosp 2009;24:369-70.
Medifood data on file 2018
Stratton RJ. et al. Disease-related Malnutrition: An Evidence-based Approach to Treatment. CABI Publishing, 2003.
Tisdale MJ et al. Cancer cachexia. Langenbecks Arch Surg 2004;389:299-305.
www.fresenius-kabi.ie accessed on accessed on 15th November 2018