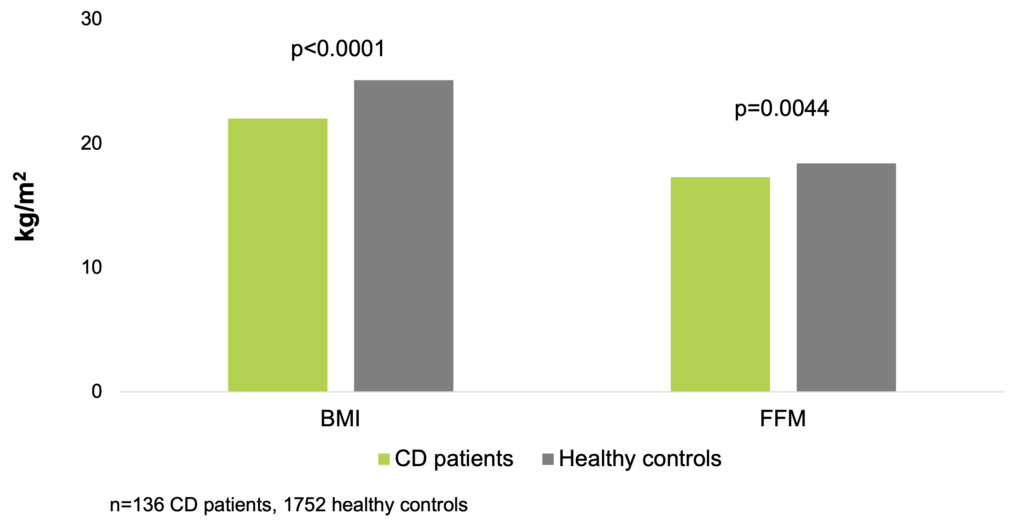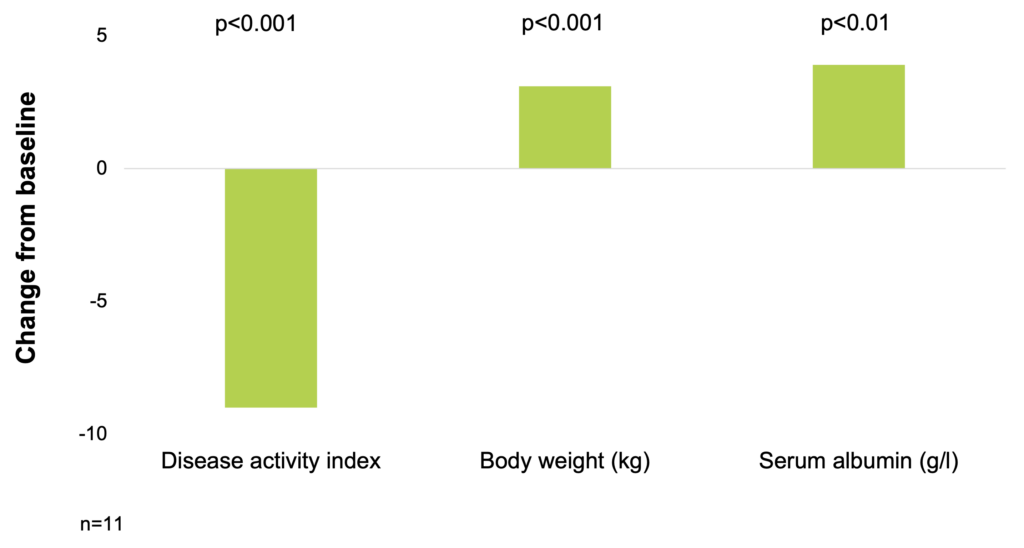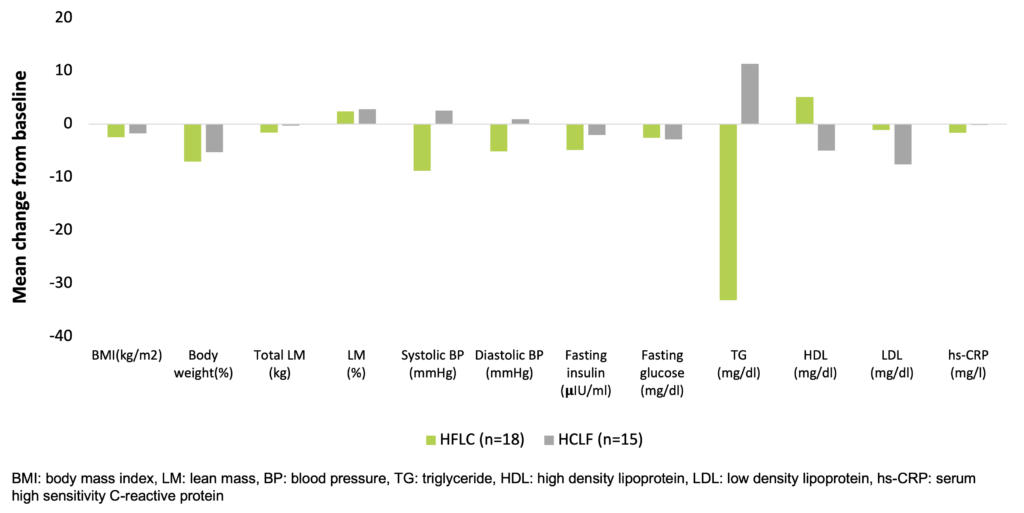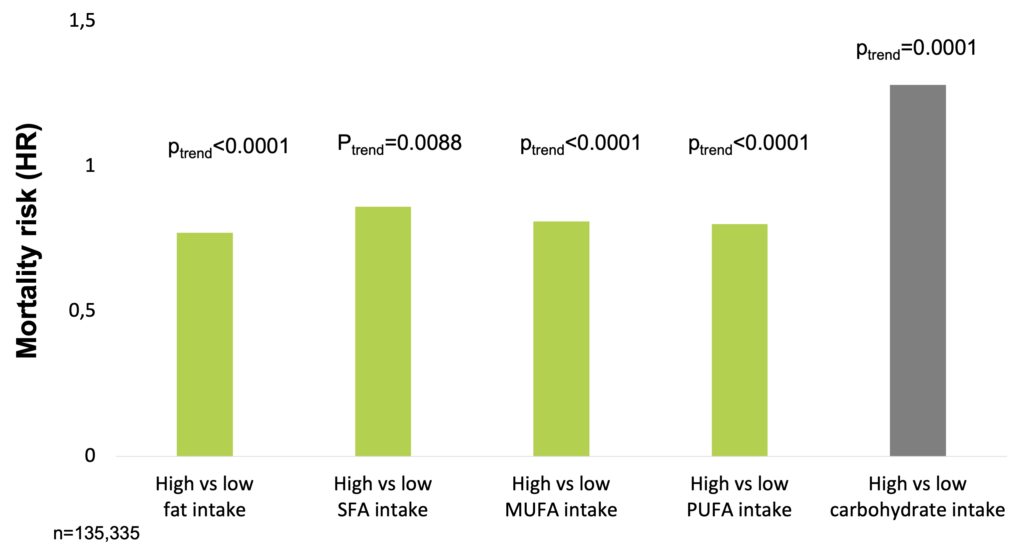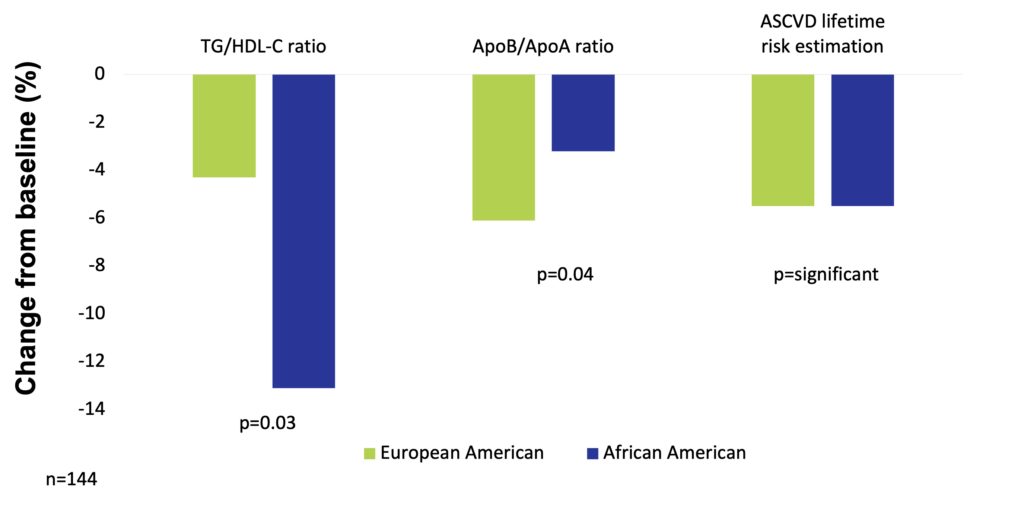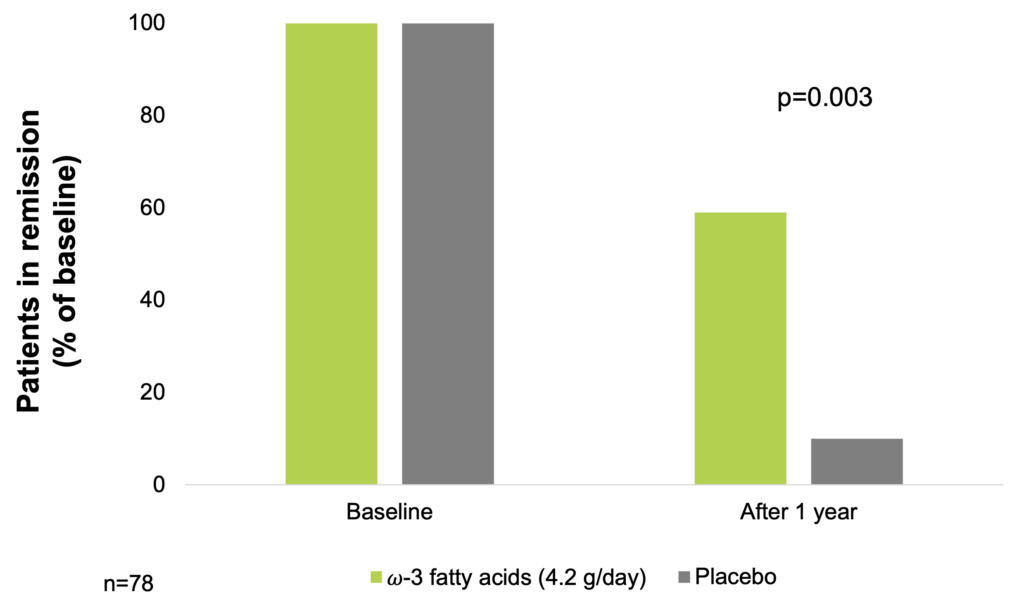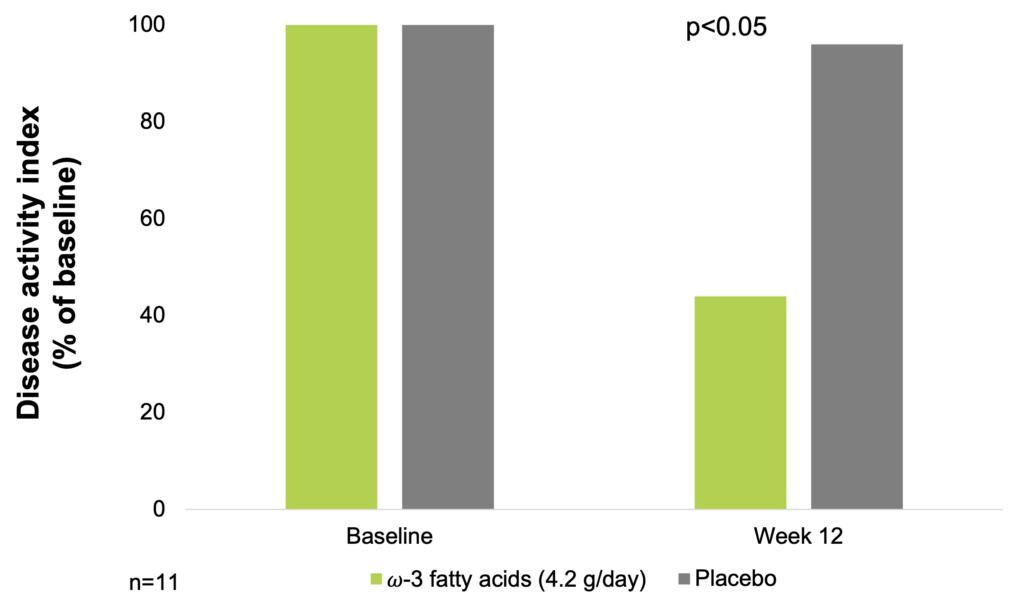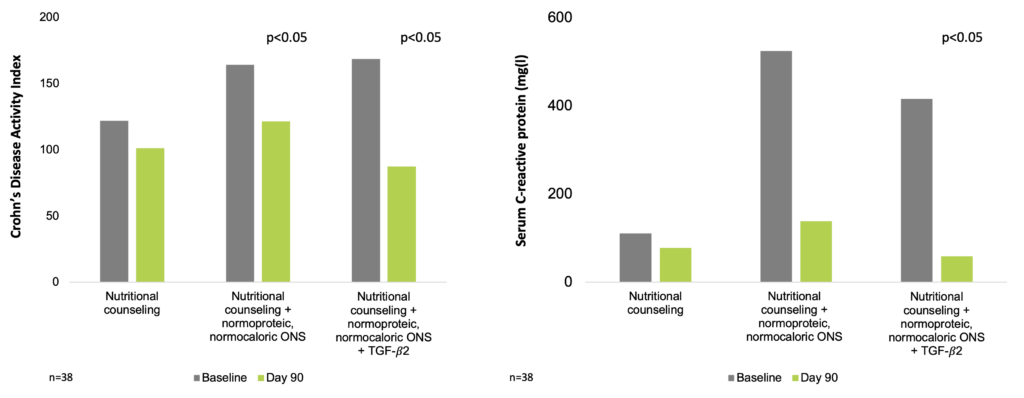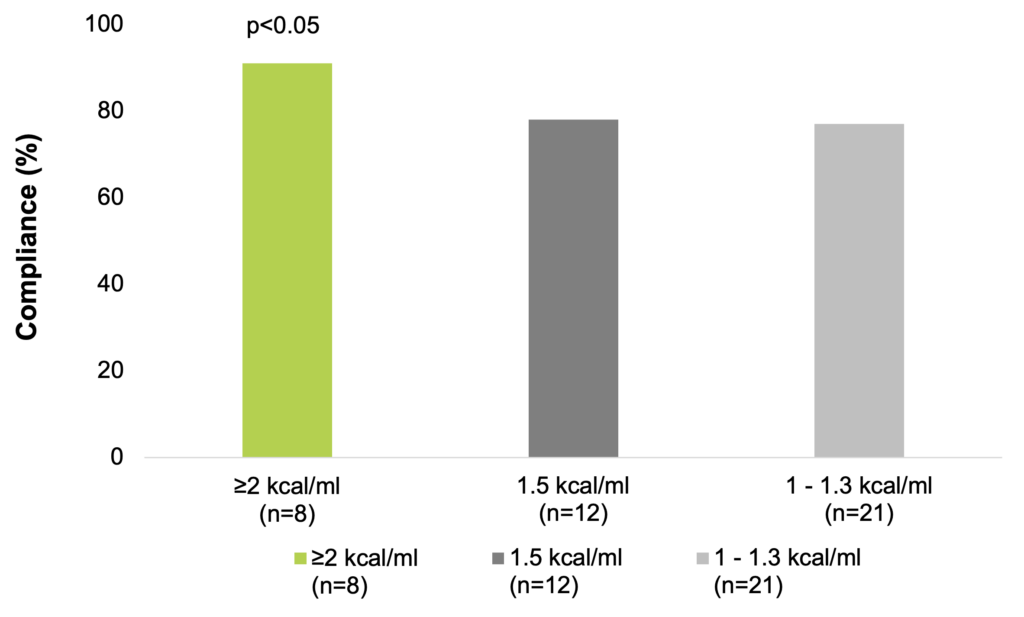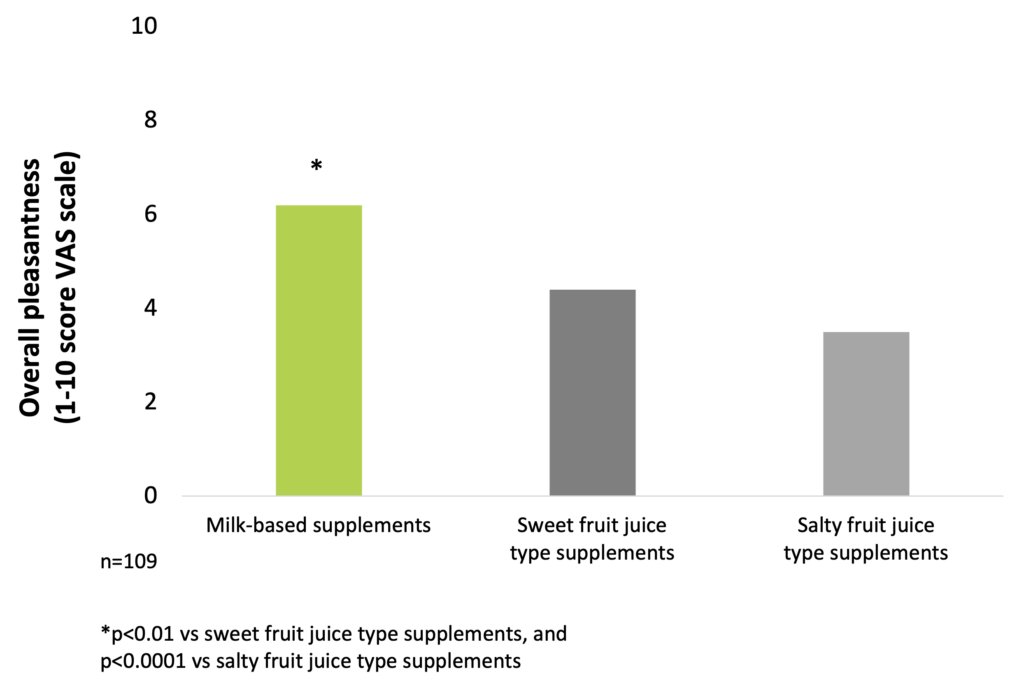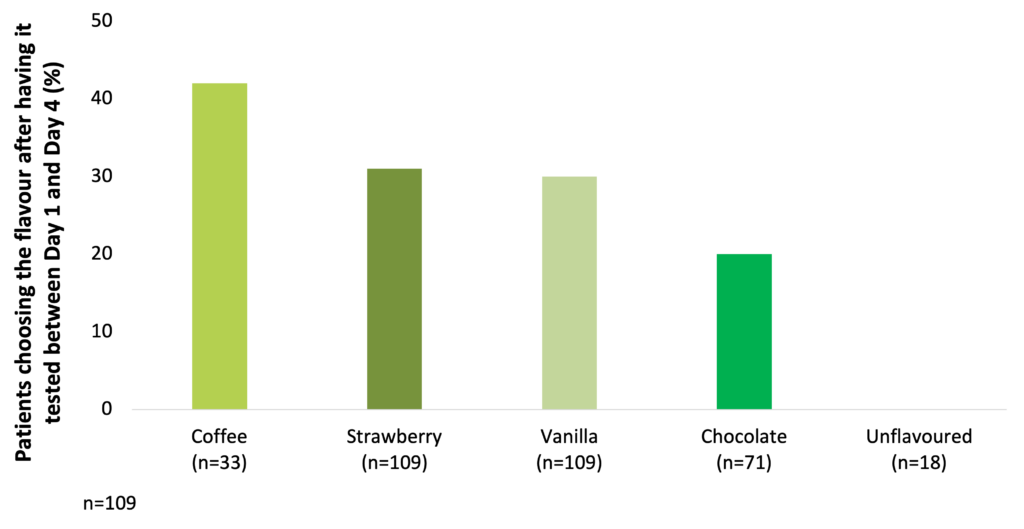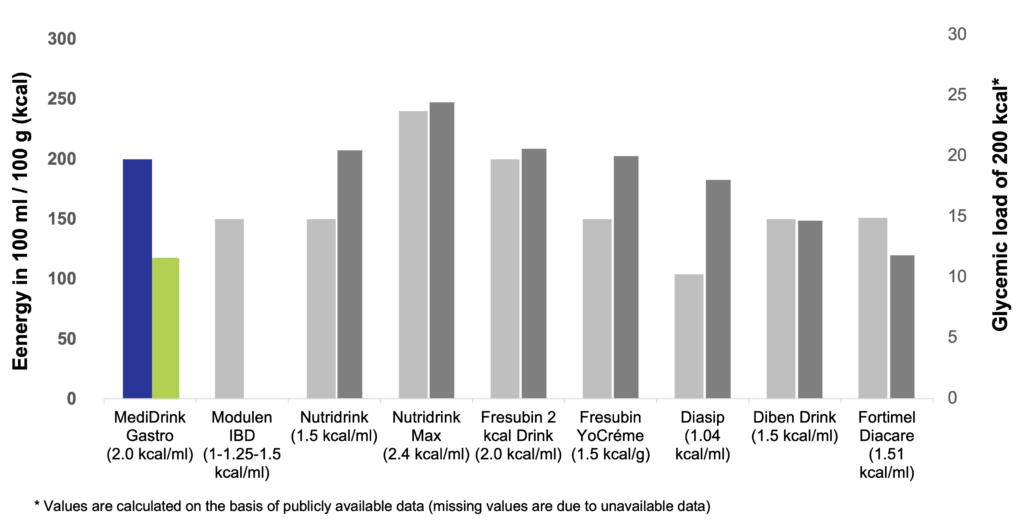MediDrink Gastro
Disease-related malnutrition
Disease-related malnutrition (DRM) has been an important and under-recognized problem for many years, affecting about 20 million patients in the EU and 33 million patients in all European countries (Ljungqvist et al. 2009, Ljungqvist et al. 2010). DRM is usually caused by the joint action of an underlying disease and dietary deficiency (Naber et al. 1997). As a consequence, treatment should focus not only on the disease itself, but also on nutritional intervention.
Malnutrition refers to deficiencies, excesses, or imbalances in a person’s intake of energy and/or nutrients. One type of malnutrition is undernutrition, which includes stunting (low height for age), wasting (low weight for height), underweight (low weight for age) and micronutrient deficiencies or insufficiencies (a lack of important vitamins and minerals). Malnutrition affects people in every country. Around 1.9 billion adults worldwide are overweight, while 462 million are underweight. An estimated 41 million children under the age of 5 years are overweight or obese, while some 159 million are stunted and 50 million are wasted (WHO 2020).
Malnutrition is a state resulting from lack of intake or uptake of nutrition, which leads to altered body composition (decreased fat free mass) and body cell mass, leading to diminished physical and mental function and impaired clinical outcome from disease. Malnutrition can result from starvation, disease, or advanced ageing (e.g., >80 years), alone or in combination (Cederholm et al. 2017).
Malnutrition is as much a cause as a consequence of ill health: poor food intake, especially for a prolonged period, makes patients more prone to illness and injury that can lead to a reduced appetite through a wide variety of mechanisms – which again results in poor food intake. Thus, it is a vicious cycle which often requires medical help to stop.
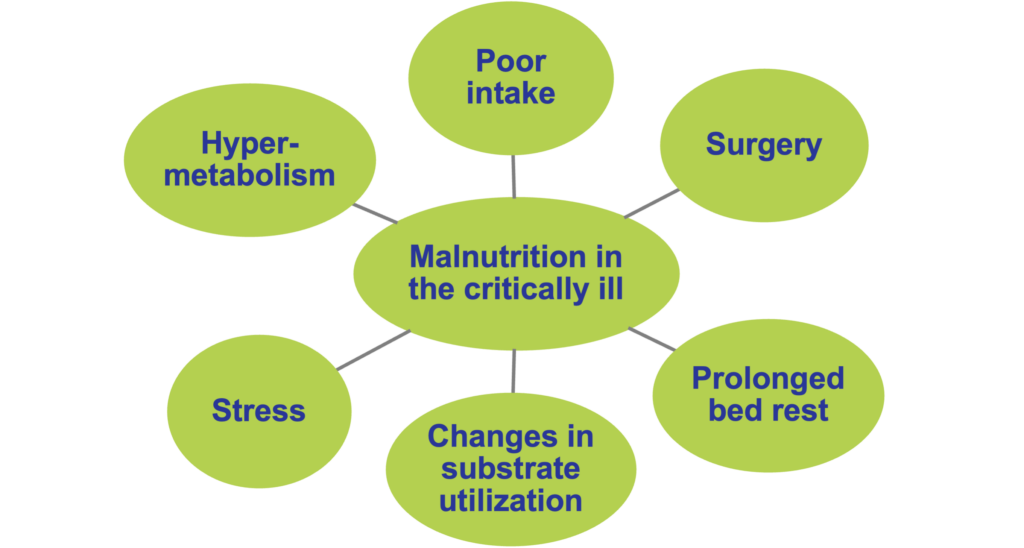
Factors contributing to the development of malnutrition in the critically ill.
According to current consensus criteria, diagnosis of malnutrition requires the presence of at least one phenotypic and at least one etiologic factor at the same time. Phenotypic factors include unwanted weight loss, low body mass index (BMI) or reduced muscle mass (based on fat-free mass index [FFMI] / bioelectrical impedance analysis [BIA] / dual-energy X-ray absorptiometry [DEXA] / computer tomography [CT] / magnetic resonance imageing [MRI] or anthropometry), whereas etiologic factors include reduced food intake or reduced food assimilation, and the presence of inflammation (either acute disease / injury-related or chronic disease-related) (Cederholm et al. 2019, Singer et al. 2019, Barazzoni et al. 2022).
Disease-related malnutrition (malnutrition caused by the changes of the body metabolism which increases the daily nutritional needs due to illness) has been an important and under-recognized problem for many years, and it continues to be a growing major public health problem with an aging population (Correia et al. 2003, Meijers et al. 2009). Since disease-related malnutrition inversely affects different organs and systems of the body and leads to severe physical and psycho-social consequences, all health care settings need to develop ways for the early diagnosis and the treatment / prevention of this condition. Surveys from the United Kingdom (UK) suggest that the majority of persons affected by or at risk of malnutrition are not those being treated in the hospital. Of the estimated 3 million people affected by malnutrition in the UK, only 2% are in hospitals, 93% live in the community, largely in their homes, 2-3% in sheltered housing, and 5% in care homes (Pryke et al. 2013). In the European Union (EU) countries, about 20 million patients are affected by disease-related malnutrition. This number is as high as 33 million patients when all countries of Europe are considered. Annual treatment costs of disease-related malnutrition reach € 120 billion in the EU, and about € 170 billion in Europe (Ljungqvist et al. 2009, Ljungqvist et al. 2010). In Germany, UK, and Ireland, the annual costs of disease-related malnutrition on a national level have been calculated: € 9 billion in 2006, and € 15 billion in 2007 (Freijer et al. 2014a, Freijer et al. 2014b).
Nutritional depletion in Western countries is usually caused by the joint action of an underlying disease (e.g. cancer, chronic obstructive pulmonary disease [COPD], inflammatory bowel disease [IBD], cognitive impairment of the elderly, Alzheimer disease) and dietary deficiency (Naber et al. 1997). As a consequence, treatment should focus not only on the disease itself, but also on nutritional intervention. The most common diseases that can cause malnutrition include oncological diseases, pulmonary diseases such as chronic obstructive pulmonary disease, and gastroenterological diseases such as inflammatory bowel disease. Certain treatments of these diseases, such as chemotherapy or radiation therapy, can also have a negative impact on nutrition.
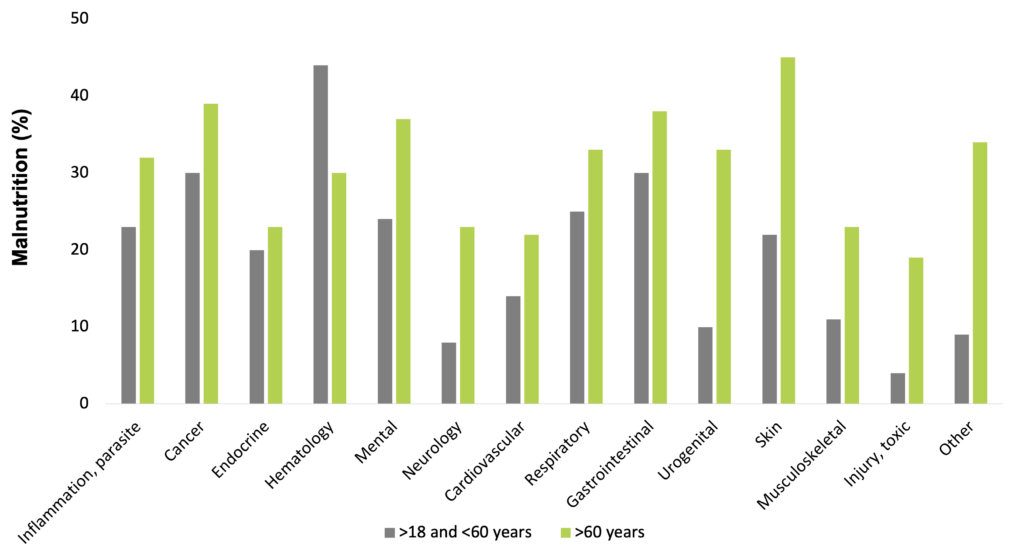
Disease-related malnutrition is present in a wide range of diseases, e.g. in infectious and parasitic diseases, oncologic, endocrine, gastrointestinal, pulmonary, hematologic, psychiatric, urogenital, and neurological disorders. The problem is known worldwide, affecting about 20 million patients with the cost of 120 billion Euros in the EU countries (Freijer et al. 2013).
Frequency of malnutrition in different diseases (Freijer et al. 2013)
For patients with a variety of diagnoses, reports indicate that up to 62% may be considered at risk of malnutrition or frankly malnourished on admission to hospital with rates of up to 12.5% in the community. Studies specifically in general medical patients indicate a possible prevalence rate of up to 56% on admission to hospital (Stratton et al. 2003).
The consequences of malnutrition, if left untreated, are serious, causing a marked decline in physical and psychological health and function. Malnutrition has a negative effect on recovery from disease, treatment efficacy, wound healing, frequency of complications (e.g. infection, decubitus), prognosis, mortality, tolerance of treatment, quality of life, and healthcare use (e.g. general practitioners visits, number and length of hospital stay) (Martyn et al. 1998, Löser C. 2010). Weight loss during an illness is a red flag for even the most inexperienced clinician, but malnutrition in the absence of manifest illness is rarely recognized as a modifiable risk factor for development of chronic diseases. The social determinants influencing food intake and hence malnutrition, e.g. loneliness and isolation, poverty, poorly fitting dentures, inaccessible food outlets, difficulty in cooking, or in supporting oneself to eat and drink, may be in operation long before associated comorbidities appear. Moreover, treatment and amelioration of these factors may delay or even prevent the onset of disease (Pryke et al. 2013).
For such a widespread significant problem, malnutrition has so far attracted very little attention in primary care. One factor of this phenomenon may be nutrition’s notable absence from most medical school curricula and postgraduate training, resulting in poor awareness, large knowledge gaps, and a deficit of nutrition-related competencies. Lack of ownership among clinicians is another factor, because malnutrition, like other processes such as pain, inflammation, and obesity, cuts across traditional clinical specialty boundaries instead of falling neatly within one or other. Reflecting this collective uncertainty and patchy knowledge, a host of unhelpful nutritional myths have also propagated and stabilized within our culture and have normalized nutritional problems. It would be timely to debunk the perception that weight loss is an inevitable part of ageing or that lower energy “healthy foods” are appropriate for everyone (Pryke et al. 2013).
There is ongoing debate in the literature about the merits of oral nutritional supplements (ONS) compared to first-line dietary advice (“food first”: information on food fortification, snacks, food choices). Skepticism regarding ONS relate to its largely hospital-focused evidence base. Issues around palatability, taste fatigue (particularly in the chronically ill requiring long-term supplementation), patient preference for “normal food”, and psychological factors were regarded as factors that might all impact the effectiveness of therapy. Thus, skeptics suggest preferring dietary advice versus ONS. Conversely, superficial dietary advice runs the risk of at-risk patients simply increasing calories without addressing essential protein and micronutrient requirements, and it is unfeasible for a “food first” approach to address nutritional deficits in some patients, particularly those with anorexia and/or early satiety. Although dietary fortification and counselling can improve nutritional intake, the evidence base is weak for improved outcomes relative to the evidence for ONS, questioning whether “food first” can replicate the combination of nutrients found in ONS (Pryke et al. 2013). These results indicate that compared to dietary counseling, the use of ONS is based on more evidence in the treatment and prevention of malnutrition. Numerous clinical studies investigated the effect of nutritional therapy on disease-related malnutrition. Meta-analyses on treatment of disease-related malnutrition with medical nutrition show a reduction in complications and mortality, improvement of wound healing, and increase of quality of life (Elia et al. 2021, Elia et al. 2005, Stratton RJ. 2005).
Malnutrition in inflammatory bowel disease
The nutritional status of inflammatory bowel disease (IBD) patients is significantly compromised at different levels (Saunders & Smith 2010, Hartman et al. 2009, Bischoff et al. 2020). Malnutrition’s prevalence ranges between 16% and 85% among Crohn’s disease patients (Mijac et al. 2010, Casanova et al. 2017, Balestrieri et al. 2020). Treatment of malnutrition should be of high importance, as it has many negative effects on the disease course of IBD (Guagnozzi et al. 2012, Bischoff et al. 2020, Mijac et al. 2010, Casanova et al. 2017, Nguyen et al. 2008, Balestrierei et al. 2020).
The major phenotypes of IBD are Crohn’s disease (CD) and ulcerative colitis (UC). The nutritional status of IBD patients is significantly compromised at different levels. There are several factors contributing to weight loss and undernutrition in CD, including the presence of chronic inflammation, low food intake, restricted diets, malabsorption, reduced absorptive surface, diarrhea, and bowel resection(s) (Saunders & Smith 2010, Hartman et al. 2009, Bischoff et al. 2020).
In the past, protein-energy malnutrition was very common in IBD patients, affecting 70-80% of hospitalized patients. Malnutrition was more common in CD than in UC patients, with an incidence ranging from 25% to 80%, especially during the remission phase. The prevalence of protein-energy malnutrition has decreased over the years but remains one of the major complications in children with IBD. Weight loss is present at diagnosis in up to 90% of children, while growth failure at the time of diagnosis has been reported in 23-88% (Guagnozzi et al. 2012). Malnutrition is common among CD patients (Bischoff et al. 2020), its prevalence ranges between 16% and 85%, depending on the assessment method used (Mijac et al. 2010, Casanova et al. 2017, Balestrieri et al. 2020).
In a clinical study of 136 CD patients and 1,752 healthy control subjects, using body BMI and FFM index (FFMI) as the markers of nutritional status, low BMI was found in 21%, and low FFMI in 30% of the patients. Low BMI values were not gender specific, but substantially more females had low FFMI values. Low BMI was diagnosed in patients vs the control group in 21% vs 4% for men and in 21% vs 2% for women, whereas low FFMI was diagnosed in 25% vs 5% for men and 36% vs 14% for women. Significant differences were found between patients and controls: median BMI 22.0 kg/m2 vs 25.1 kg/m2, p<0.0001; FFMI: 17.3 kg/m2 vs 18.4 kg/m2, p=0.0044. This study confirmed the higher prevalence of low FFMI than that of low BMI among the subjects (Molnar et al. 2017).
Median BMI and FFMI are significantly lower in CD patients (Molnar et al. 2017)
According to data from a study of 41 patients with IBD, intake of protein (p<0.05), carbohydrate (p<0.05), fiber (p<0.05), iron (p<0.05), sodium (p<0.05), potassium (p<0.05), zinc (p<0.01), vitamin B6 (p<0.05), vitamin C (p<0.05), and folate (p<0.01) were significantly lower in the malnourished group. The intakes of fiber, calcium, vitamin B2, vitamin C, and folate were lower than the recommended nutritional intakes in the normal group. The intakes of fiber, calcium, zinc, vitamin B1, vitamin B2, niacin, vitamin C, and folate were lower in the malnourished group than the recommended nutritional intakes (Heesook et al. 2014).
Treatment of malnutrition should be of high importance, as it has many negative effects on the disease course of IBD: it negatively affects disease prognosis, increases the risk of thromboembolic, infectious, and postoperative complications (e.g., anastomotic failure) (Guagnozzi et al. 2012, Bischoff et al. 2020, Mijac et al. 2010), increases hospital admission rates (Bischoff et al. 2020), increases hospital length of stay (Bischoff et al. 2020, Casanova et al. 2017, Nguyen et al. 2008), decreases patients’ quality of life (Bischoff et al. 2020, Casanova et al. 2017, Balestrierei et al. 2020, Nguyen et al. 2008), and increases mortality (Bischoff et al. 2020, Nguyen et al. 2008). Moreover, undernutrition causes humoral and cellular immunodeficiency, leading to impairment of the mucosal barrier and a greater risk of infection by bacterial translocation (Guagnozzi et al. 2012).
Clinical nutrition in IBD
If the gut can be used safely, enteral nutrition is the preferred feeding method for patients with CD and UC (Guagnozzi et al. 2012). A high protein semi-elemental diet improves nutritional status in patients with active CD (Ferreiro et al. 2021).
Cachexia is a complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass. The prominent clinical feature of cachexia is weight loss in adults (corrected for fluid retention) or growth failure in children (excluding endocrine disorders). From an energy point of view, an increased weight loss may be related to a decrease in thermodynamic efficiency. Variable thermodynamic efficiency in metabolic systems is related not only to differences in weight but also may confer metabolic advantages or drawbacks. At present, it is widely held that elevated resting energy expenditure (REE) is a major determinant in the development of malnutrition in cachectic patients. Resting energy metabolism represents the combustion of fuel sources needed to provide energy for metabolic processes involved in maintaining the function and integrity of cells and body organs and for the mechanical processes involved in keeping the body alive. It is appropriate, therefore, to presume that abnormalities in carbohydrate, lipid, and protein metabolism are major biochemical bases of elevated REE (Hyltander et al. 1991, Argiles et al. 2014).
Treatment for cachexia has concentrated on increasing food intake, although that alone is unable to reverse the metabolic changes (Tisdale et al. 2004). Due to the hypercatabolic state and increased protein breakdown seen in malnourished / cachectic patients, increased energy and protein intake is vital in slowing down / stabilizing or even reversing weight loss and lean body mass /muscle wasting in cachectic patients (Cawood et al. 2012, Broekhuizen et al. 2005, Guagnozzi et al. 2012).
If the gut can be used safely, enteral nutrition is the preferred feeding method for patients with CD and UC. The advantages of enteral nutrition include its stimulatory effects on the gastrointestinal structure and function as well as its reduced cost compared to parenteral feeding. Enteral nutrition seems to exert a direct anti-inflammatory effect on the intestinal mucosa by reducing interleukin (IL)-6 production and increasing insulin-like growth factor (IGF)-1 production. Enteral nutrition has been proven to be effective in the treatment of the acute phase of CD, achieving remission rates from 20% to 84.2% regardless of disease location (Guagnozzi et al. 2012).
In a study of 11 patients with severe refractory CD, a high calorie, whole diet induced remission in 8 patients with a decrease in disease activity score from 13 to 4 (p<0.001) and a reversal of subacute obstruction in all cases. All patients had a weight gain with a mean increase of 3.1 kg after treatment (p<0.001). The weight gain was paralleled with an increase in the arm muscle area, and also in the triceps skinfold thickness. The serum albumin level improved with treatment and increased by a mean of 3.9 g/l (p<0.01). The induction of remission paralleled the improvement in nutritional parameters suggesting that improved nutrition may be the important factor affecting disease activity (Afdhal et al. 1989).
A high calorie, whole diet induced remission in patients with severe refractory Crohn’s disease (Afdhal et al. 1989)
The different protein sources can influence the antigen recognition or presentation process and may induce or maintain chronic intestinal inflammation (Guagnozzi et al. 2012). An in vitro study aimed to determine whether elemental diet or its modifications have a direct anti-inflammatory effect on colonic tissue biopsies. Colonic or ileal biopsies from 39 patients with IBD and control patients were incubated for 24 hours with enteral diets in which nitrogen sources were amino acids as in elemental diet, casein, or whey, at dilutions of 1:5, 1:10 or 1:20. IL-1𝛽, IL-1 receptor antagonist and IL-10 concentrations in supernatants were measured by immunoassay. Elemental diet incubation increased anti-inflammatory : proinflammatory cytokine ratio in CD and this anti-inflammatory effect was not specifically due to amino acid composition, as diets containing casein had similar anti-inflammatory effects (Meister et al. 2002).
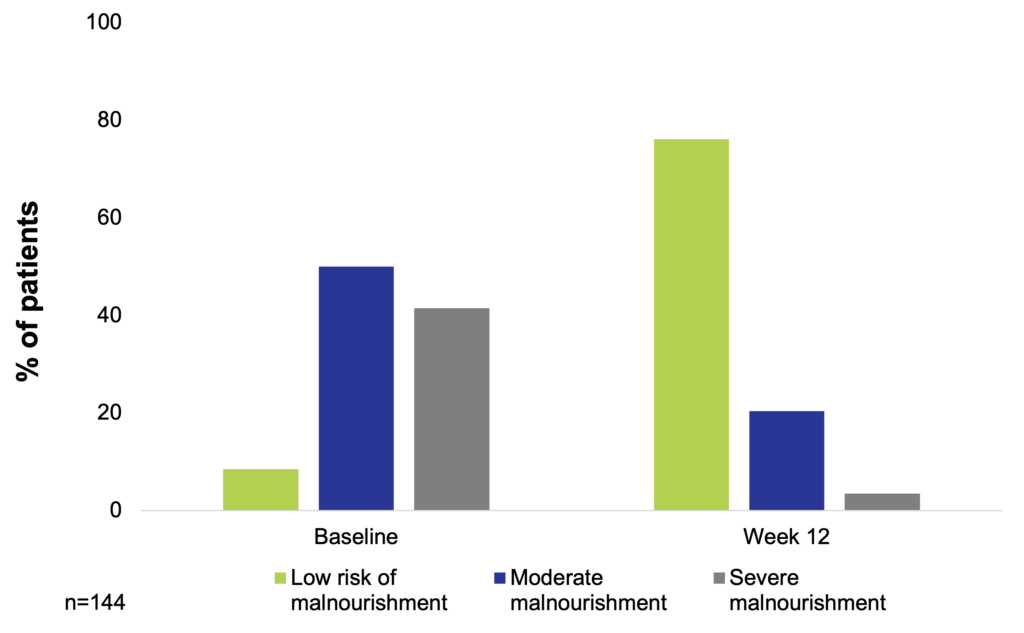
An observational, prospective study assessed the nutritional status, disease activity, and stool frequency at baseline and after 12 weeks of treatment with a high-protein semi-elemental diet in patients with active CD. A total of 144 patients with CD were included. The nutritional status improved after treatment, resulting in 76.1% of patients being at low risk of malnourishment, 20.4% being moderately malnourished, and 8.5% being severely malnourished after 12 weeks of treatment. Nutritional status improvement was associated with the number of nutritional supplements consumed. Mean albumin levels and BMI improved after 12 weeks of nutritional treatment (from 3.0 g/dl to 3.7 g/dl and from 20.2 kg/m2 to 21.1 kg/m2, respectively). A significant decrease in the Harvery-Bradshaw index was found after 12 weeks of nutritional treatment (from 10.2 to 3.7). The mean number of stools per day decreased with the 12-week semi-elemental diet (from 4.6 stools/day to 1.7 stools/day) (Ferreiro et al. 2021).
A high protein diet can improve nutritional status in patients with active Crohn’s disease (Ferreiro et al. 2021)
Low carbohydrate nutrition induces improvement in mucosal and clinical symptoms in CD patients (Lutz et al. 1986), and reduces the growth of Lactobacilli and its consequences in patients with short small bowel (Bongaerts & Severijnen. 2006).
Enteral nutrition is beneficial for patients with IBD, but the source of energy is also of crucial importance. According to a study of 103 CD patients, low carbohydrate nutrition induced improvement in mucosal and clinical symptoms. After half a year on the low-carbohydrate nutrition, more than 60% of the patients were asymptomatic, after one year more than 70%, and after one and a half year about 85%. Of 74 UC patients treated with a low-carbohydrate diet, approximately 60% of the patients were without complaints after 2 years, had normal laboratory values, and a normal-looking rectal mucosa. The remaining 40% also achieved remission after a treatment period of longer than 2 years (Lutz et al. 1986).
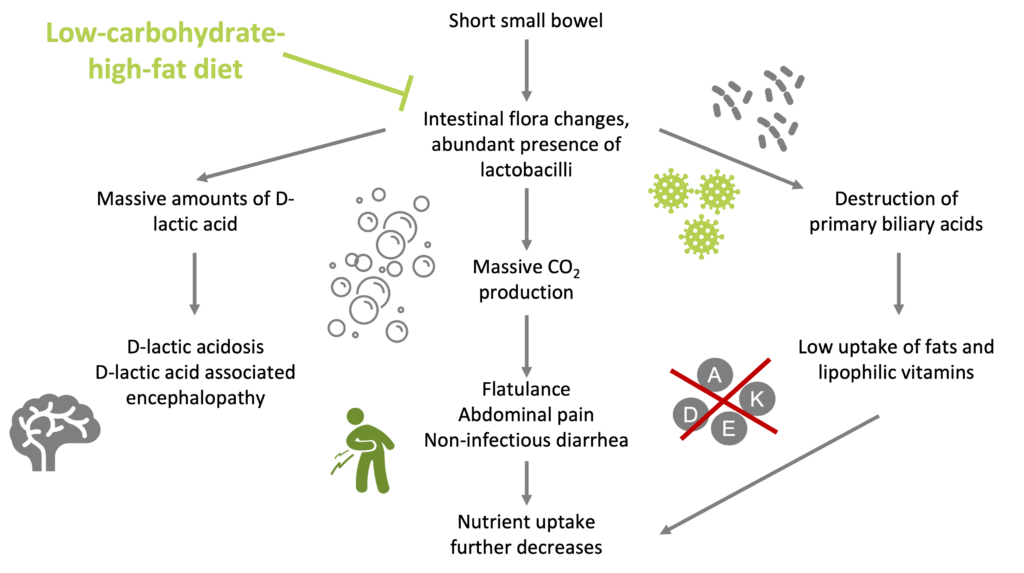
Despite the availability of powerful immunosuppressants, many patients with CD still require one or more intestinal resections throughout the course of their disease. Multiple resections and a progressive reduction in bowel length can lead to the development of short small bowel syndrome (SSB) (Limketkai et al. 2016). SSB patients suffer from malabsorption due to a strongly reduced small bowel surface. These patients usually get a high-energy-high-carbohydrate-low-fat diet at oral or enteral feeding. In these patients, the intestinal flora is strongly changed and even becomes characteristic due to abundant presence of Lactobacilli (up to nearly 100%). In many patients with a high-carbohydrate-low-fat diet, these bacteria both produce massive amounts of D-lactic acid and gaseous CO2, and they destroy the primary bile acids that are necessary for the uptake of lipids. Thus, this diet may cause (i) an increased risk of D-lactic acidosis and D-lactic acid-associated encephalopathy, (ii) flatulence, abdominal pain, and non-infectious diarrhea, and (iii) low uptake of fat, and lipophilic vitamins. It is argued that by gradually converting the diet to a low-carbohydrate-high-fat one, growth of the characteristic lactobacilli can be strongly reduced as well as the mentioned inconveniences (Bongaerts & Severijnen. 2006).
Low-carbohydrate-high-fat diet strongly reduces the growth of Lactobacilli and its consequences in patients with short small bowel (Bongaerts & Severijnen. 2006)
Safety of a low carbohydrate diet
Larosa et al. were the first to demonstrate in 1980 (and confirmed by others many times since) that low-carbohydrate diets (30-130 g/day) do not necessarily require higher fat or protein intakes, and a spontaneous decrease in overall calorie consumption frequently results in little protein or fat added back for the carbohydrate removed (Larosa et al. 1980). This causes the phenomenon that a very low carbohydrate diet ameliorates all the investigated anthropometric laboratory parameters (BMI, abdominal fat, triglycerides, HDL, triglycerides/HDL ratio, ApoB/ApoA1 ratio, small LDL, blood glucose, plasma insulin, saturated fatty acid) of patients with atherogenic dyslipidemia (Hite et al. 2011). In a study, which randomized obese subjects (29.0-44.6 kg/m2) recruited from Boston Medical Center to a hypocaloric low-fat-high-carbohydrate / LFHC (n=26) or high-fat-low-carbohydrate / HFLC (n=29) diet for 12 weeks, the HFLC group had greater improvements in blood lipids and systemic inflammation with similar changes in body weight and composition, relative to the LFHC group (Ruth et al. 2013).
Beneficial effects of a low-carbohydrate diet in obese subjects (Ruth et al. 2013)
In a large, epidemiological cohort study of individuals aged 35–70 years in 18 countries with a median follow-up of 7.4 years (IQR 5.3-9.3), dietary intake of 135,335 individuals was recorded using validated food frequency questionnaires. Participants were categorized into quintiles of nutrient intake (carbohydrate, fats, and protein) based on percentage of energy provided by nutrients. During follow-up, 5,796 deaths and 4,784 major cardiovascular disease events were documented. Higher carbohydrate intake was associated with an increased risk of total mortality (highest [quintile 5] vs lowest quintile [quintile 1] category, HR=1.28 [95% CI 1.12-1.46], ptrend=0.0001) but not with the risk of cardiovascular disease or cardiovascular disease mortality. Intake of total fat and each type of fat was associated with lower risk of total mortality (quintile 5 vs quintile 1, total fat: HR=0.77 [95% CI 0.67-0.87], ptrend<0.0001; saturated fat [SF], HR=0.86 [0.76-0.99], ptrend=0.0088; monounsaturated fat [MUFA]: HR=0.81 [95% CI 0.71-0.92], ptrend<0.0001; and polyunsaturated fat [PUFA]: HR=0.80 [95% CI 0.71-0.89], ptrend<0.0001). Higher saturated fat intake was associated with lower risk of stroke (quintile 5 vs quintile 1, HR=0.79 [95% CI 0.64-0.98], ptrend=0.0498). Total fat and saturated and unsaturated fats were not significantly associated with risk of myocardial infarction or cardiovascular disease mortality. Based on these results, the authors suggest the reconsideration of global dietary guidelines (Dehghan et al. 2017).
In a large, epidemiological cohort study, high carbohydrate intake was associated with higher risk of total mortality, whereas total fat and individual types of fat were related to lower total mortality (Dehghan et al. 2017).
One hundred and forty-four premenopausal women of age 21-50 years with class I/II obesity (BMI 30-39.9 kg/m2) were recruited in a study to keep a balanced high-fat diet (50% fat, 30% carbohydrate, 15% protein, with a balanced fat content – 1/3 saturated fatty acids, 1/3 monounsaturated fatty acids, 1/3 polyunsaturated fatty acids) for 16 weeks. To control the effects of high simple carbohydrate intake, total carbohydrate was maintained as 50% sugars (mono- and disaccharides) and 50% starches. Results in European American and African American participants were analyzed separately. Consuming the balanced high-fat diet significantly reduced cardiovascular risk by 5.5% (increased HDL particle size, increased the number of large HDL particle size, and increased apolipoprotein AI level) in both groups. In addition, European American women had significant reductions in fasting insulin levels (by 24.8%), and in HOMA-insulin resistance (by 29%). In the group of European American women, the most significant improvements occurred in VLDL particle size, apolipoprotein B levels, serum triglyceride, number of plasma LDL particles, and serum LDL cholesterol (Niswender et al. 2018).
High-fat balanced diet improves atherosclerotic cardiovascular disease risk in obese premenopausal women (Niswender et al. 2018)
ω-3 fatty acids supplementation has several beneficial effects in CD and UC (Wiese et al. 2011, Sung et al. 2013, Belluzzi et al. 1996, Aslan A. & Triadafilopoulos G. 1992, Scaioli et al. 2018). With its anti-inflammatory effects (Rautava et al. 2011), milk-derived transforming growth factor (TGF)-β enriched nutrition reduces disease activity index and CRP levels in CD patients (Ferreira et al. 2020).
ω-3-polyunsaturated fatty acids (PUFAs) are considered to have a beneficial effect in the management of IBD by repressing cytokine production and modulating the production of eicosanoids. ω-3-PUFAs repress the activity of leukocytes, proliferation and synthesis of inflammatory cytokines (e.g. tumor necrosis factor / TNF, IL-1), activity of natural killer cells, synthesis of antibodies as well as the expression of surface membrane molecule of macrophages. It is proposed that ω-3 fatty acids contribute to the modulation of the inflammatory process and can act even as scavengers for free radicals (Ioannidis et al. 2011).
Epidemiological studies have shown that the recent increase in the incidence of CD is strongly related to the increased uptake of ω-6-PUFAs, zoic milk protein, and the quotient of ω-6:ω-3 consumption (Ioannidis et al. 2011).
In an open-label pilot study of 28 patients with active CD, the consumption of nutritionally balanced IBD nutrition formula enriched with ω-3 fatty acids resulted in significantly lower CD activity index (116 ± 94.5 vs 261.8 ± 86.5; p=0.005) and higher IBD questionnaire score (179.1 ± 26.6 vs 114.6 ± 35.9, p<0.001) when plasma eicosapentaenoic acid (EPA) concentration was >2% compared to <2% (Wiese et al. 2011).
In a 1-year double-blind, placebo-controlled study of 78 patients with CD with high possibility of recurrence, effect of supplementation with 2.7 g/day fish oil (2/3 EPA and 1/3 docosahexaenoic acid / DHA) was investigated. After 1 year, 23 patients (59%) in the fish oil group remained in remission, as compared with 10 patients (26%) in the placebo group (p=0.003). Monovariant statistical analysis demonstrated that the fish oil was exclusively responsible for the decrease of recurrence possibility compared with the placebo group (OR=4.2, 95% CI: 1.6 – 10.7) (Belluzzi et al. 1996).
ω-3 fatty acids supplementation may elongate remission in CD (Belluzzi et al. 1996)
A meta-analysis of 9 randomized, placebo-controlled trials of fish oil (a total of 1.8-6.2 g/day of EPA + DHA or total ω-3 fatty acids) administered for at least 6 months to IBD patients showed that the pooled relative risk of CD relapse was significantly decreased (RR=0.77, 95% CI: 0.61-0.98) (Sung et al. 2013, Turner et al. 2011), though a funnel plot analysis suggested publication bias for the smaller studies included (Turner et al. 2011).
Studies in UC animal models showed that the use of balanced diets containing ω-3 long-chain PUFA could ameliorate the inflammation and the mucosal damage (Nieto et al. 2002), the protective effects of fish oil are mediated through molecular mechanisms involving the redox regulation of metabolism of key lipid metabolites (Sharma et al. 2019), and that the simultaneous administration of sodium 2-mercaptoethane sulfonate and ω-3 PUFAs is particularly effective in ameliorating dextran sodium sulphate (DSS)-induced colitis in rats, by reducing oxidative stress, inflammation and apoptosis, probably through a mechanism that involves the inhibition of NF-κB and overexpression of iNOS (Triantafyllidis et al. 2016). Treating animals with walnut oil in a DSS-induced colitis mouse model improved symptoms of inflammation and sustained the intestinal barrier in the distal colon (Bartoszek et al. 2020). Dietary supplementation with walnut had the ability to protect mice against DSS-induced colitis (Nakanishi et al. 2019), while perilla oil treatment improved colitis in the DSS-induced mouse model, indicating its potential role in managing conditions such as UC (Thomas et al. 2022).
In a prospective cohort study of 25,639 men and women aged 45–74 years (EPIC-Norfolk), information was collected on diet using detailed 7-day food diaries, which had been validated against 16-day weighed records and biological markers. A total of 22 incident cases of UC were identified of which 45% (n=10) were in women. The median age at diagnosis was 72.1 years (range 48.1–80.8 years). In the analysis, each case was matched with four randomly selected controls of the same sex, date of birth (±6 months) and date of recruitment into EPIC (±3 months) and who were alive at the time when the matched case was diagnosed. The nutrient data was 100% complete for all cases and controls. An increasing dietary intake of DHA was associated with a statistically significant decrease of risk of developing incident UC when adjusted for cigarette smoking and total energy intake. The effect size for the highest tertile of DHA intake was associated with a 79% (95% CI: 17–95%) decreased risk of UC with a statistically significant trend across tertiles (OR=0.47, 95% CI: 0.25–0.89, p=0.02). These negative associations for DHA persisted when the analyses were adjusted for other fatty acids that may influence the metabolism of DHA (OR for trend across tertiles=0.43, 95% CI: 0.22–0.86, p=0.02). The proportion of cases of UC, which could be prevented if all cases consumed the highest tertiles of intake of DHA was 57% (John et al. 2010).
In a 6-month, open-label, randomized, placebo-controlled trial, 53 adult UC patients in clinical remission randomized to an Anti-Inflammatory Diet (AID) that had a structured four-week menu plan including recipes and nutrition tips, intake of zinc, phosphorus, selenium, yogurt, and seafood was increased compared to that of the control group randomized to a diet based on Canada’s Food Guide (CFG). Five patients (19.2%) in the AID and 8 patients (29.6%) in the CFG group experienced a clinical relapse. The subclinical response to the intervention (defined as fecal calprotectin <150 μg/g at the endpoint) was significantly higher in the AID group (69.2 vs. 37.0%, p=0.02) (Keshteli et al. 2022).
In an 8-month, double-blind, placebo-controlled, crossover trial of dietary supplementation with fish oil, 11 patients with UC of mild to moderate severity received about 4.2 g of ω-3-fatty acids per day. Disease activity index based on patient symptoms and sigmoidoscopic appearance was used to assess efficacy of ω-3 fatty acid-treatment of UC. Mean disease activity index declined by 56% for patients receiving fish oil and by 4% for patients on placebo (p<0.05) (Aslan A. & Triadafilopoulos G. 1992).
ω-3 fatty acids supplementation improves disease activity index in ulcerative colitis (Aslan A. & Triadafilopoulos G. 1992)
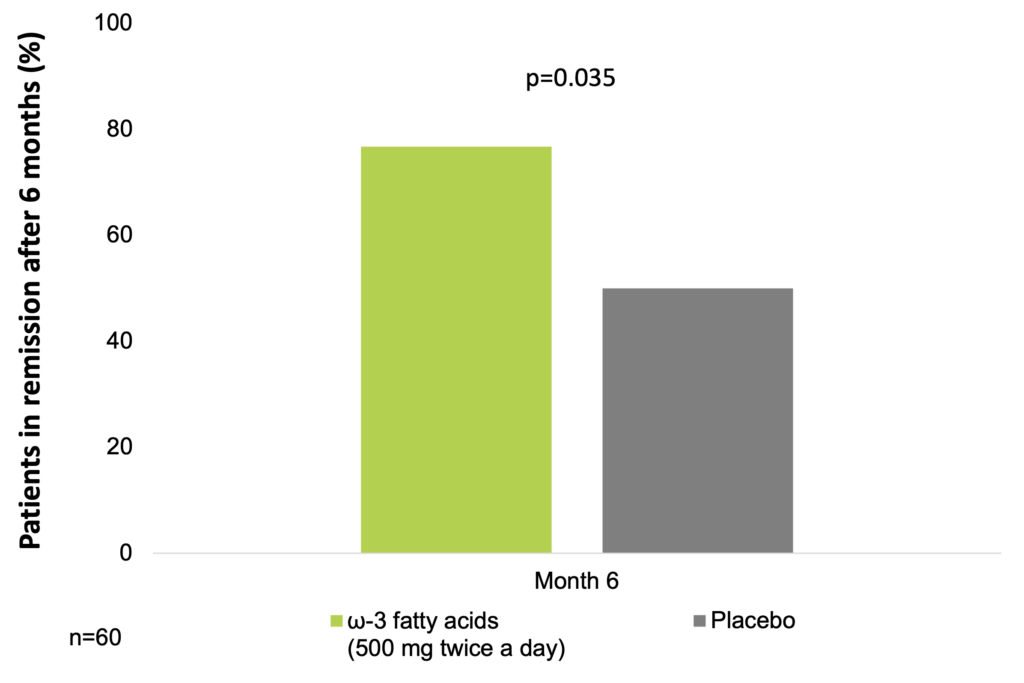
In a placebo-controlled trial, 60 patients with UC with a partial Mayo score <2 and fecal calprotectin ≥150 μg/g, on stable therapy for at least the 3 previous months, were randomly assigned (1:1) to groups given either the free fatty acid form of EPA (EPA-FFA) 500 mg twice daily, or placebo for 6 months. The primary end point of a 100-point reduction in fecal levels of calprotectin at 6 months from the baseline value was achieved by 19 of 30 patients (63.3%) in the EPA-FFA group vs 4 of 30 patients (13.3%) in the placebo group (OR=12.0; 95% CI: 3.12–46.24; p<0.001). The secondary endpoint of maintenance of clinical remission at 6 months was achieved by 23 of 30 patients (76.7%) in the EPA-FFA group vs 15 of 30 (50%) patients in the placebo group (OR=3.29; 95% CI: 1.08–9.95; p=0.035). No serious adverse events were observed (Scaioli et al. 2018).
ω-3 fatty acids supplementation may help sustain remission in UC (Scaioli et al. 2018)
In 20 initial-onset IBD patients who had not undergone any dietary intervention, supplementation with an ω-3 food exchange table significantly increased the ω-3/ω-6 [EPA/arachidonic acid (AA)] ratio via a decrease in the erythrocyte membrane ω-6 PUFA level and an increase in the ω-3 PUFA level. Among the UC and CD patients, the ω-3/ω-6 (EPA/AA) ratio in the remission group was significantly higher. According to the authors, to maintain IBD remission, the erythrocyte membrane ω-3/ω-6 ratio should be kept at 0.65 or higher. Furthermore, the percentage weight of the erythrocyte membrane EPA should be maintained at 2.55% or greater (Uchiyama et al. 2010).
In a randomized crossover study of 9 patients with mild or moderate active UC, addition of 4.5 g/day fish oil ω-3 fatty acids to the 2 g/day sulfasalazine therapy for 2 months significantly decreased the oxidative stress measured by the total plasma antioxidant capacity, lipid peroxidation inhibition and superoxide dismutase levels (Barbosa et al. 2003).
In a Mendelian randomization analysis of single nucleotide polymorphisms involving 114,999 individuals in UK Biobank, and 20,833 CD and 27,432 UC patients, ω-3 fatty acids were observed to have a protective effect against UC (He et al. 2022).
The milk-derived transforming growth factor (TGF)-β is a pleiotropic cytokine/growth factor with effects ranging from regulation of cell proliferation and differentiation to modulation of adaptive immune responses. In the intestine, TGF-β is involved in regulating inflammatory responses, establishing oral tolerance by regulatory T cells, inducing IgA production and enhancing the intestinal epithelial barrier function. TGF-β1 is the predominant TGF-β isoform produced by immune cells, whereas TGF-β2 is most abundant in secretions including milk (Rautava et al. 2011).
In an animal study, IL-10 -/- mice fed a TGF-β2-containing diet gained more weight, developed no diarrhea or prolapse, had lower pathological scores, and lower serum amyloid A levels. These data support the use of TGF-β2-containing enteral diets as one mode of therapy for CD (Oz et al. 2004).
In 3 studies examining the value of a TGF-β-enriched formula in patients with CD, the patients received the TGF-β diet for 8 weeks as their sole source of nutrition, followed by a 4-week-period of controlled reintroduction of normal food. The TGF-β diet was effective in inducing remission and mucosal healing. Biochemical markers of inflammation, erythrocyte sedimentation rate and C-reactive protein (CRP) levels normalized, and serum albumin levels improved significantly. Endoscopic examination revealed a significant improvement also in the appearance and histology of the mucosal tissue. In addition, there was a reduction in the mRNA levels for the pro-inflammatory cytokines IL-1β, IL-8 and interferon (IFN)-γ (Hartman et al. 2008).
In a prospective study of 38 CD patients, subjects were allocated into 3 groups: group 1 (patients received only nutrition orientation), group 2 (nutrition orientation and a normoproteic, normocaloric nutrition supplement), and group 3 (nutrition orientation and nutritional supplement with TGF-β2). Clinical and nutrition evaluation, CRP levels, and assessment of endoscopic and histologic parameters in the intestinal mucosa were performed before and after nutrition intervention. The mean follow-up period was 3 months. In the beginning of the study, groups were homogeneous regarding age, gender, CD behavior and localization, and medication in use. In the end of the study, the Crohn’s Disease Activity Index (CDAI) score was reduced in groups 2 and 3. In group 3, a reduction in CRP levels and an improvement in histologic findings were also observed. Among patients who received nutritional supplement, some anthropometric patterns were improved. The results of the study indicated that nutritional supplementation improved nutrition and inflammatory patterns in patients with active CD. However, only patients receiving a TGF-β2–enriched formula showed improvement in histologic parameters and significant reduction in CRP levels (Ferreira et al. 2020).
TGF-β2 may help reduce inflammation in CD patients (Ferreira et al. 2020)
Medium chain triglycerides (MCTs) are lipids that are utilizable as energy source even in the presence of fat malabsorption (Sankararaman & Sferra 2018), and they may also exhibit immune-modulatory properties (Cabre & Domenech. 2012).
Medium-chain triglycerides (MCTs) are esters of glycerol and fatty acids with a carbon chain consisting of 6-12 carbon atoms (Sankararaman & Sferra 2018, Lee et al. 2022). MCTs are absorbed more easily from the small intestinal lumen than long chain triglycerides, as their absorption is independent of bile salts and pancreatic lipases (Sankararaman & Sferra 2018). Unlike other lipids, MCTs are transported to the portal system rather than the mesenteric lymph after absorption (Sankararaman & Sferra 2018, Lee et al. 2022). Once in the liver, MCTs undergo hepatic 𝛽-oxidation and provide energy instantaneously – this metabolic pathway is therefore independent of glucose/insulin signaling (Sankararaman & Sferra 2018, Lee et al. 2022). As a consequence, MCTs are lipids that are utilizable as energy source even in the presence of fat malabsorption (Sankararaman & Sferra 2018).
Although MCTs are traditionally considered as mere easy-to-oxidize energy sources, recent data support the idea that they can also exhibit immune-modulatory properties. In fact, there is growing experimental evidence that MCTs are able to improve bowel damage both in spontaneous and induced animal models of intestinal inflammation. In an animal model of IBD (trinitrobenzene sulfonic acid ileitis model), the mucosal damage series in the MCT group was significantly lower than in the long chain triglycerides (LCT) group, and levels of TNF-α and leukotriene B4 tended to be lower in the MCT group. These results suggest that MCT modulates intestinal inflammation and is less damaging than LCT (Tsujikawa et al. 1999). There are also some clinical data suggesting that replacing part of dietary fat with MCT would contribute to the primary therapeutic effect of enteral nutrition in CD (Cabre & Domenech. 2012).
Glutamine and arginine are amino acids thought to play important roles in the maintenance of intestinal tissue integrity (Kim & Kim 2017, Fritz 2013).
Glutamine and arginine are amino acids thought to play important roles in the maintenance of intestinal tissue integrity (Kim & Kim 2017, Fritz 2013). It has been proposed that glutamine is involved in several important processes in the physiology of intestinal epithelial cells, such as regulation of enterocyte proliferation, maintenance of tight-junction proteins, modulation of inflammatory pathways, and protection against apoptosis and cellular stresses (Kim & Kim 2017).
Arginine is also implicated in different functions of intestinal epithelial cells, including the regulation of intestinal barrier function, and decreasing intestinal inflammation (Fritz 2013).
Chronic zinc (Zn) deficiency increases the production of pro-inflammatory cytokines (Bonaventura et al. 2015). Adequate dietary selenium supply may counteract chronic intestinal inflammation in humans (Speckmann & Steinbrenner. 2014), and supports protective gut microbiota which indirectly improves management of IBD (Ala & Kheyri. 2021).
Zinc (Zn) functions as a modulator of the immune response through its availability, which is tightly regulated by several transporters and regulators. When this mechanism is disturbed, Zn availability is reduced, altering survival, proliferation, and differentiation of the cells of different organs and systems, and, in particular, cells of the immune system. Zn deficiency affects cells involved in both innate and adaptive immunity at the survival, proliferation, and maturation levels. These cells include monocytes, polymorphonuclear-, natural killer-, T-, and B-cells. T cell functions and the balance between the different T helper cell subsets are particularly susceptible to changes in Zn status. While acute Zn deficiency causes a decrease in innate and adaptive immunity, chronic Zn deficiency increases inflammation. During chronic Zn deficiency, the production of pro-inflammatory cytokines increases, influencing the outcomes of a large number of inflammatory diseases (Bonaventura et al. 2015).
Inadequate dietary intake of the essential trace element selenium (Se) is thought to be a risk factor for several chronic diseases associated with oxidative stress and inflammation. Biological actions of Se occur through low-molecular weight metabolites and through selenoproteins. Interestingly, Se and antioxidant selenoproteins are known to modulate differentiation and function of immune cells and to contribute to avoiding excessive immune responses (Speckmann & Steinbrenner. 2014). Several key selenoproteins, including glutathione peroxidases; selenoproteins M, P, and S; and selenium-binding protein 1; have been detected in the intestine. In humans, Se deficiency is commonly observed in patients with CD. In animal models of experimental colitis, Se status was negatively correlated to the severity of the disease. The beneficial outcome of dietary Se supplementation and an optimization of selenoprotein biosynthesis in murine IBD models have led to investigations of targets and actions of Se in the gastrointestinal tract. Se status affects gene expression, signaling pathways, and cellular functions in the small and large intestine. These data, particularly from animal experiments, hold promise that adequate dietary Se supply may counteract chronic intestinal inflammation in humans (Speckmann & Steinbrenner. 2014).
Furthermore, Se supports protective gut microbiota which indirectly improves management of IBD. Se supplementation also showed promising results in preliminary clinical studies particularly in patients with Se deficiency (Ala & Kheyri. 2021).
Exclusive enteral nutrition
Exclusive enteral nutrition (EEN) is recommended as first line therapy for induction of remission in children 17 years or younger with active luminal CD (Bischoff et al. 2020, Ashton et al. 2019, van Rheenen et al. 2020), with a remission rate between 60% and 80% (Bischoff et al. 2020, Ashton et al. 2019, van Rheenen et al. 2020). When compliance is not an issue, EEN may also be helpful in adult CD (Mitrev et al. 2021).
During exclusive enteral nutrition (EEN), 100% of the daily calorie intake is provided in the form of a liquid formula-based diet (Ashton et al. 2019, van Rheenen et al. 2020).
EEN is recommended as first line therapy for induction of remission in children 17 years or younger with active luminal CD (Bischoff et al. 2020, Ashton et al. 2019, van Rheenen et al. 2020). EEN has many positive attributes: efficacy of EEN is comparable to that of corticosteroid (CS) therapy (Yu et al. 2019), with a remission rate between approximately 60% and 80% (Bischoff et al. 2020, Ashton et al. 2019, van Rheenen et al. 2020), EEN can also induce mucosal healing (van Rheenen et al. 2020, Yu et al. 2019), 12-month relapse rates of EEN are similar to that of CS therapy (van Rheenen et al. 2020, Yu et al. 2019), while EEN has only a few side effects when compared to CSs, and most are described as not serious (Day & Lopez 2015, Luo et al 2015, de Sire et al. 2021). These side effects are most commonly of gastrointestinal nature, such as nausea, diarrhea, constipation, bloating and abdominal pain (Day & Lopez 2015, Luo et al 2015). The most serious side effect of EEN is refeeding syndrome; however, it is rare (Day & Lopez 2015). To avoid refeeding syndrome, screening for malnutrition must be done before commencing EEN, and once commenced in a malnourished individual, the caloric intake should be gradually increased to the desired target value. Close monitoring of plasma electrolyte levels is also of utmost importance in malnourished patients receiving EEN (Day & Lopez 2015).
CS therapy in children is frequently associated with growth-retarding effects, a side effect rarely associated with the use of EEN. Compared to CS, a reduced incidence of linear growth failure can be seen with EEN (7% vs 26% with CSs), while EEN also ensures complete food intake and can correct macro- and micronutrient deficiencies (van Rheenen et al. 2020).
The average duration of EEN for induction of remission is around 6-8 weeks, and the number of calories to be provided is estimated based on weight and age (Ashton et al. 2019, van Rheenen et al. 2020). Further positive attributes of EEN include more weight gain than that seen in patients undergoing CS therapy (Yu et al. 2019), and a higher probability of decreasing Pediatric Crohn’s Disease Activity Index (PCDAI) score compared to CS therapy (3.7 times more likely to reduce PCDAI compared to CS) (Yu et al. 2019).
Adult CD patients are generally less compliant with EEN than are children (Mitrev et al. 2021). The major reasons for noncompliance include poor palatability of some formulations, lack of variety, missing out on the social aspects of food, therapy associated financial costs, and the length of treatment (Mitrev et al. 2021).
Limited evidence suggests that when compliance is not an issue, EEN may also be helpful in adult CD (Mitrev et al. 2021). A prospective observational study found that EEN induced complete mucosal healing in 23 out of 29 (79%) adult CD patients (Chen et al. 2019). The mean time to reach mucosal healing was 123 days (range 50-212 days) in this patient population (Chen et al. 2019)
MediDrink Gastro
MediDrink Gastro is a 2.0 kcal/ml, complete, lactose- and gluten-free food for special medical purposes for the the dietary management of active phase of Crohn’s disease as a sole source of nutrition and dietary management of inflammatory bowel diseases as nutritional support.
MediDrink Gastro is not suitable for children below 3 years of age, and for patients with galactosemia and hereditary fructose intolerance (fructosemia). The dosage of MediDrink Gastro administered should be decided by a physician.
MediDrink Gastro is available in 3 different flavors: chocolate, strawberry, and vanilla.
Compliance, safety, tolerability
Compliance is significantly greater to ≥2 kcal/ml ONS compared to standard 1.5 kcal/ml ones (Hubbard et al. 2012). Overall pleasantness of taste for patients is significantly better for milk-based supplements (Darmon et al. 2008).
MediDrink Gastro is suitable also for diabetic IBD patients (Medifood Data on file 2022).
Compliance
According to data from a meta-analysis, the overall compliance to ONS is good, especially with higher energy-density ONS, resulting in improvements in patients’ total energy intakes that have been linked to clinical benefits. A range of ONS-related attributes have been associated with compliance. Significantly greater compliance and energy intakes are seen with the use of small volume, energy dense products (energy content ≥2 kcal/ml) compared to standard 1.5 kcal/ml ONS. Therefore, in patients who struggle to ingest the prescribed volume of ONS, a change to a higher energy density ONS could increase total energy intake and reduce wastage. This strategy could be employed in the nutritional management of malnourished individuals (Hubbard et al. 2012).
Compliance is greater with higher energy-density ONS (Hubbard et al. 2012)
A randomized controlled interventional trial of 77 elderly nursing home residents with high functional impairment showed that a low-volume, nutrient- and energy-dense ONS was well accepted and resulted in significant improvements of nutritional status and, thus, was effective to support treatment of malnutrition (Stange et al. 2013).
Taste
The investigation of taste preferences of milk-based and fruit juice-type supplements in malnourished in-patients showed that the overall pleasantness is significantly better for milk-based supplements than for sweet and salty fruit juice-type products (Darmon et al. 2008).
Pleasantness of milk-based ONS is significantly better than that of sweet and salty fruit juice-type products (Darmon et al. 2008)
Among milk-based ONS, coffee, strawberry, vanilla, and chocolate are the most preferred flavours (Darmon et al. 2008).
Coffee, strawberry, vanilla, and chocolate are the most preferred flavours among milk-based ONS (Darmon et al. 2008)
Glycemic load
The concept of glycemic load (GL) has been introduced by Walter Willett at the Harvard School of Public Health in 2004. While glycemic index (GI) ranks the different sources of carbohydrates according to their blood sugar-raising capacity, GL takes also into account the quantity of food / carbohydrates consumed. GL is calculated by multiplying the GI of the carbohydrates by the quantity of the carbohydrates taken in. Therefore, GL of a certain food depends not only on the GI but also the quantity consumed (Venn & Green 2007, Fajcsák & Lelovics. 2006). Based on the calculation of GL, MediDrink Gastro has the lowest GL and therefore the lowest strain on the metabolism of the patients among the different ONS depicted below. Thus, MediDrink Gastro is suitable also for diabetic patients, since when consuming the same amounts of kilocalories, the GL of MediDrink Gastro is lower than that of the ONS specifically designed for diabetic patients.
Energy content and glycemic load of several oral nutritional supplements
(Medifood data on file 2022, https://www.nestlehealthscience.co.uk/sites/default/files/2020-07/modulen-datacard-july-2019.pdf accessed on 23rd November 2023, [Complete therapy with clinical nutrition] Nutricia 2016, http://www.fresenius-kabi.co.uk/ (accessed on 15th November 2018), www.fresenius-kabi.ie accessed on accessed on 15th November 2018, https://www.fresubin.be/fr-be/produits/fresubin-db-drink/ accessed on 7th November 2023)
References
[Complete therapy with clinical nutrition] – Nutricia, 15th June 2016.
[ONS index] 2014. Vidal Hungary Kft.
Argiles JM. et al. Cachexia: a problem of energetic inefficiency. J Cachexia Sarcopenia Muscle 2014;5:279-86.[NE1]
Balestrieri P. et al. Nutritional Aspects in Inflammatory Bowel Diseases. Nutrients 2020;12:372.
Bonaventura P. et al. Zinc and its role in immunity and inflammation. Autoimmun Rev 2015;14:277-285.
Elia M. et al. To screen or not to screen for adult malnutrition? Clin Nutr 2005;24:867-84.
Fajcsák Zs. and Lelovics Zs. [Glycemic index and glycemic load]. Új Diéta 2006;4:28-9.
Freijer K. et al. The economic costs of disease related malnutrition. Clin Nutr 2013;32:136-41.
Fritz JH. Arginine cools the inflamed gut. Infect Immun 2013;81:3500-2.
Hite AH. et al. Low-carbohydrate diet review: shifting the paradigm. Nutr Clin Pract 2011;26:300-8.
http://www.fresenius-kabi.co.uk/ (accessed on 15th November 2023)
https://www.nestlehealthscience.co.uk/sites/default/files/2020-07/modulen-datacard-july-2019.pdf accessed on 23rd November 2023
https://www.fresubin.be/fr-be/produits/fresubin-db-drink/ accessed on 7th November 2023
Ljungqvist O. et al. The European fight against malnutrition. Clin Nutr 2010;29:149-50.
Ljungqvist O. Man FD. Under nutrition: a major health problem in Europe. Nutr Hosp 2009;24:369-70.
Medifood data on file 2022
Sankararaman S. & Sferra TJ. Are We Going Nuts on Coconut Oil? Curr Nutr Rep 2018;7:107-115.
Saunders J. & Smith T. Malnutrition: causes and consequences. Clin Med (Lond) 2010;10:624-7.
Stratton RJ. et al. Disease-related Malnutrition: An Evidence-based Approach to Treatment. CABI Publishing, 2003.
Tisdale MJ et al. Cancer cachexia. Langenbecks Arch Surg 2004;389:299-305.
www.fresenius-kabi.ie (accessed on accessed on 15th November 2023)